When approaching the synthesis of an aryl- or heteroaryl- amine most people would turn to a suitable transition metal (Pd, Cu or Ni) catalysed C-N cross-coupling reaction- most likely a Buchwald-Hartwig reaction, or, if this has limited success, Ullmann or Chan-Evans-Lam couplings.1 Photoredox approaches using aryloxy amides as a source of amidyl radicals began a trend in moving away from transition metals and more recently catalytic redox cycling of main group elements offers a complementary approach to these important pharmaceutical and agrochemical intermediates.2 In a recent blog post I described a paper by McNally on the synthesis of heterobiaryl compounds using a phosphorus(V) contractive C-C coupling reaction- an alternative to the Suzuki-Mayara coupling.3 Another group led by Alex Radosevich at MIT have targeted aryl C-N cross-coupling chemistry and recently described an organophosphorus catalysed C-SP2-H intramolecular amination of ortho-nitrobiaryls (Cadogan cyclization).4 The Radosevich group have now extended the scope of this work to include intermolecular coupling of nitroarenes with boronic acids using the same redox-recycling (PIII/PV=O) phosphorus catalyst (J. Am. Chem. Soc. 2018, 140(45), 15200)- Figure 1.
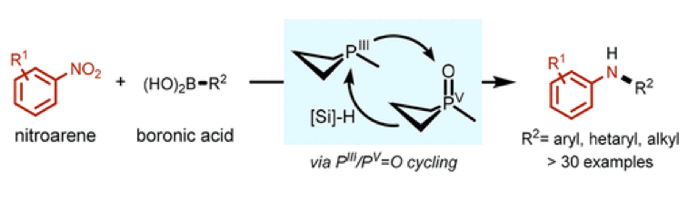
Figure 1: P-mediated reductive C-N cross coupling of nitroarenes and boronic acids
Conversion of PIII to PV=O is a strong driving force for many synthetic transformations.5 A limitation of these methods is poor atom economy and the generation of stochiometric quantities of phosphine oxide waste. Recently several groups have addressed this challenge and published catalytic methods in which cycling of the phosphine oxide intermediates through redox-neutral or redox-driven modes is achieved.6 The Radosevich group have focused heavily on development of biphilic catalysts which unify the nucleophilic donor and electrophilic acceptor reactivity of the phosphorus atom (Figure 2).4 They have demonstrated that a small-ring phosphacycle (typically a core 4-membered ring system) in combination with a phenylsilane terminal reductant promotes reductive O-transfer from a substrate nitroarene by cycling in the PIII / PV=O couple.
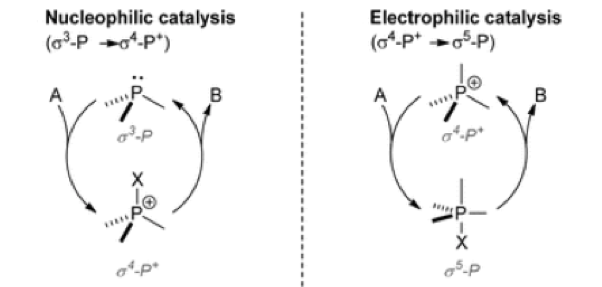
Figure 2: biphilic organophosphorus compounds utilize the s5-P phosphoranes for catalysis
The Radosevich group used the reaction of nitrobenzene with phenylboronic acid as suitable baseline process for optimisation and started with the same conditions reported for the intramolecular Cadogan cyclisation (59% yield, 1 eqv nitrobenzene, 1.5 eqv phenylboronic acid, 20 mol% phosphine oxide, 2 eqv PhSiH3, toluene, 100°C, 16hrs, Figure 3) .4
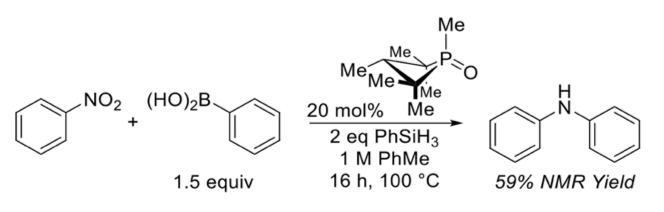
Figure 3: initial coupling conditions
A DoE approach, evaluating temperature, concentration and reagent stoichiometry in m-xylene solvent increased the isolated yield to over 80% (1 eqv nitrobenzene, 1.1 eqv phenylboronic acid, 15 mol% phosphine oxide, 2 eqv PhSiH3, 0.5M m-xylene, 120°C, 4 hrs). A comparable yield was obtained using the PIII phosphetane confirming that cycling to PV=O was occurring. Use of stochiometric phosphetane (3 eqv) again gave comparable results. Control experiments omitting silane reductant and phosphorus catalyst gave no conversion. The nature of the boron source was important- boronic esters gave low yields of coupled product compared to boronic acid or cyclic boroxine trimers.
Electronic demands for both coupling partners revealed election-withdrawing p-substitution on the nitroarene promoted a faster rate of reaction and higher yields of cross coupling products, with a complimentary inverse empirical demand for donating substituents in the boronic acid intermediate. This is a key differentiating observation over palladium catalysed C-N cross couplings where arylation of electron deficient aryl amines are challenging. PIII/PV=O coupling may provide a future path forward for construction of deactivated C-N bonds.
The paper describes examples of various substituted aryl- and heteroaryl- nitro compounds and boronic acids, including a few alkyl-boronic acids. The latter tend to give lower yields, as one might expect. As is the modern trend with methodology papers a couple of drugs were prepared to demonstrate utility. Mefenamic acid and tolfenamic acid (NSAIDs marked as Ponstel and Clotam respectively) were prepared in one pot (two step) processes in good yield on a 1 mmol scale.
The reaction was demonstrated to be stereospecifc with respect to the boronic acid component enabling future application in asymmetric synthesis.
This is an interesting area and is sure to be of interest to the organocatalysis community. I look forward to reading more from the Radosevich group.
References:
- C-N cross coupling references: Ullmann: Soc. Rev. 2014, 43, 3525; ibid 2013, 42, 9283; Chan-Lam: Tetrahedron 2012, 68, 7735; Pd-catalysed: Chem. Rev. 2016, 116, 12564; J. Am. Chem. Soc., 2017, 139, 4769; N-Radical couplings: Adv. Synth. Catal. 2018, 360, 2076; J. Am. Chem. Soc. 2016, 138, 8092.
- Opin. Chem. Biol. 2010, 14, 347-361.
- Heterocyclic phosphonium salts- powerful intermediates for pyridine coupling
- J. Am. Chem. Soc.2017, 139, 6839; ibid 2018, 140, 3103. For other examples using PIII to PV=O cycling see Angew. Chem. Int. Ed. 2019, 58, 2864; J. Am. Chem. Soc. 2015, 137, 616; ibid 2015, 137, 5292.
- Phosphorus chemistry generating P=O as a by-product: Wittig, Appel, Mitsunobu.
- Catalytic phosphorus review articles; Catalytic witting reaction review: Beilstein J. Org. Chem. 2016, 12, 2577–2587; Phosphine Organocatalysis Rev. 2018, 118, 10049; Development of a catalytic Witting reaction see Chem. Eur. J. 2013, 19, 15281; Catalytic Appel: Chem. Eur. J. 2011, 17, 11290. Towards a catalytic Mitsunobu reaction: Org. Bimol. Chem. 2018, 16, 7774.








