Heterocyclic phosphonium salts- powerful intermediates for pyridine coupling:- I’ve always had a keen interest in phosphorus chemistry and have followed the work of the McNally group at Colorado state on heterocyclic phosphonium salts closely. In late 2016 a paper describing synthesis and application of the so called HetPhos salts for C4 (or C2) functionalisation of pyridines was published (Figure 1).[1]
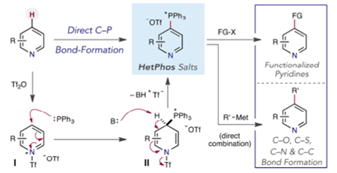
Figure 1
These stable, free-flowing solids were prepared by reaction of a suitable pyridine (including late stage drug-like molecules) with Tf2O at low temperature, followed by sequential addition of PPh3 and an organic base. Reaction with nucleophiles, particularly alkoxides but also azide, alkylthiol and lithium heteroaryls, gave good yields of the 4-pyridyl derivatives. More recently a collaboration with Merck process R&D showed that phosphonium salts derived from azaarenes, upon reaction with potassium carbonate and a deuterium or tritium source (CD3OD/D2O or THO) gave good yields of the C4 isotopically labelled derivatives (Figure 2).[2] Again, a number of late-stage bioactive molecules were exemplified.[3]
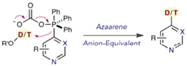
Figure 2
The latest application of these versatile intermediates is an alternative approach to metal catalysed cross coupling chemistry to make heterobiaryls using a phosphorus (V) contractive C-C coupling reaction (Science 2018, 362, 799). The heterobiaryl pharmacophore is commonly encountered in drug receptor binding, examples including Gleevec and the Cox-2 inhibitor Etoricoxib. The key C-C bond is invariably made via metal catalysed cross coupling of aryl halides with suitable coupling partners using Pd, Ni or Cu.[4] The reported phosphorus-based coupling relies on sequential formation of a C-P bond with the azaarene, generating the bis-azaarene phosphonium salt, and final C-C bond formation via contraction of a P(V) intermediate (3a, Figure 3). The final biaryl coupling step (3a to 4a) was initiated using acidic ethanol at 80°C. Phosphorus-ligand coupling is a known process and can also be triggered using alkoxides and organometallics such as Grignard reagents. Significantly, heteroaryl-phenyl or bis-phenyl products were not observed in the reaction.
The paper describes extensive mechanistic studies on the phosphorus-ligand coupling, including both experimental and computational investigations. The azaarene scope is exemplified, including late stage coupling of advanced intermediates in the synthesis of complex biomolecules. Gleevec and Etoricoxib are represented. Practically, the salt formation needs to be carried out is a specific order.[5] Reassuringly the team established a set of robust guidelines and limitations. Reagents are readily available, and the conditions appear relatively simple. Time will tell if this method becomes a player in the cross-coupling arena. This could be the advent of a move away from heavy metal methodology.
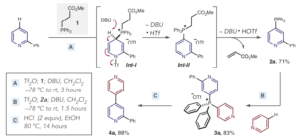
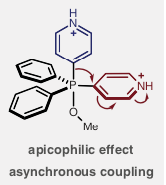
Figure 3
[1] McNally etal J. Am. Chem. Soc. 2016, 138, 13806
[2] A similar intermediate and mechanism is reported for transfer of an Ar group from Ph3PAr+ to aldehydes and ketones. See Nature Communications 7, 2016, 10337
[3] McNally etal J. Am. Chem. Soc. 2018, 140, 1990
[4] Large scale applications of transition metal-catalysed couplings for the synthesis of pharmaceuticals Chem. Rev.2011, 111, 2177
[5] Supplementary material: Click here








