It’s not often that drug molecules find themselves on the reagent shelf alongside materials that may have been used to assemble them. The prince and the pauper so to speak. In 2019 Paul Hergenrother from the University of Illinois published a paper in Angewandte Chemie claiming a diazomethane surrogate that was a commercially available, weighable, solid: non-explosive and non-toxic, with application in esterification and cyclopropanation reactions.1 The remarkable thing about this compound is that it is a billion-dollar drug, Temozolomide (TMZ, Figure 1), and is currently the standard of care for the treatment of gliobalastoma and a second-line treatment for recurrent anaplastic astrocytoma.2
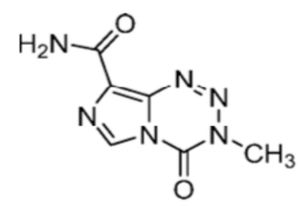
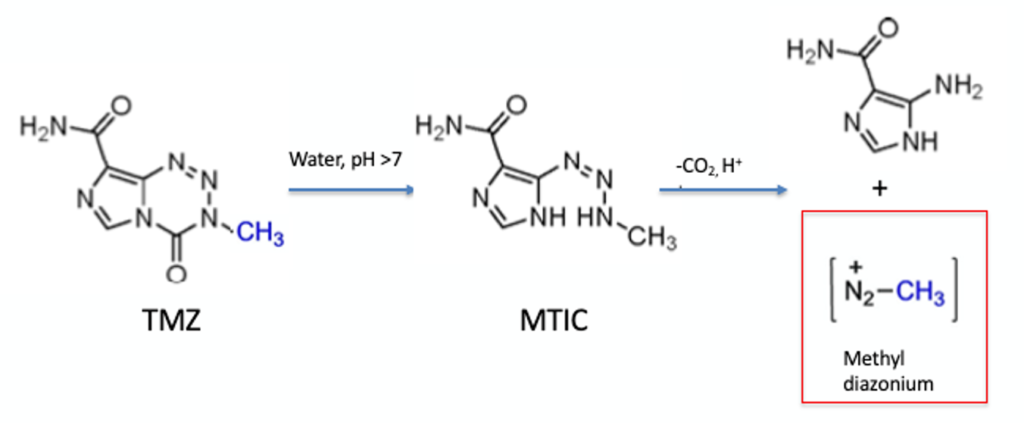
The molecule contains a 1,2,3,5-tetrazin-4-one motif, one of several reactive heterocycles investigated in the 1980’s by Malcom Stevens and Robert Stone at Aston University.3 TMZ hydrolyses rapidly at physiological pH (7.4), ring-opening to a triazene intermediate (MTIC), which then rapidly releases the powerful methylating agent diazomethane in vivo (Figure 2). Methylation of DNA induces cell cycle arrest at the G2/M phase inducing apoptosis and killing cancerous cells.
Synthetically, highly toxic and explosive diazomethane gas (b.p. -23°C) is generated in situ using agents such as diazald (or more water-soluble structural variants), and used directly as an ethereal solution (Figure 3). Alternatively, TMS-diazomethane is used as a safer, non-explosive, surrogate (though it most likely generates diazomethane as the active methylating agent in situ). More recently generation and handling of diazomethane has been carried out under continuous processing conditions- particularly at scale.4 Phoenix chemicals, based in the UK (now obsolete), were making aspartate-derived a-haloketones for me on multi-kilo scale using a continuous diazomethane generator back in the early 2000’s. The benefit of continuous generation is that there is always a low inventory of the hazardous reagent at any one time so that if the worst should happen the collateral damage is minimised. At Phoenix diazomethane was produced and consumed in a continuous process capable of generating between 50 and 60 tonnes per year whilst the maximum inventory was maintained at less than 80g.5
At the time of reading the Angewandte paper I remember feeling somewhat sceptical of TMZ’s apparent thermal stability- the authors claiming the material was non-explosive and stable up to its melting point of 212°C. These are bold claims to make without extensive testing. If the data is not readily available, or even if it is published in some form, it needs to be verified and re-verified to establish any batch-to-batch variation or process impurities that could lower any decomposition onset temperature. That gauntlet was recently taken up by Jeff Sperry and his team at my alma mater Vertex.6
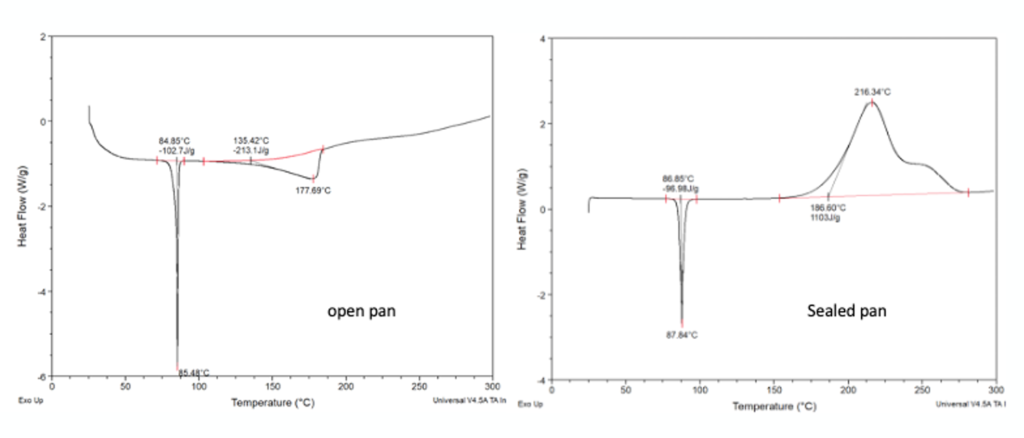
The workhorse of thermal screening and initial safety evaluation is the differential scanning calorimeter (DSC). All process chemistry labs should have access to one of these machines and should have standard testing procedures for any new compounds, reagents, or reaction mixtures to see how they behave under controlled heating conditions. Although academic groups will often publish DSC data in the literature, thermal hazard assessment is often only conducted in unsealed crucibles (with no conditions reported) which could (and very often does) lead to misleading results and dangerously incorrect conclusions.7 Often gas evolution will mask a significant exotherm in an open crucible and can appear as an endothermic event. A good example of this point is the recently described triazene-based diazo transfer reagent ADT (2-azido-4,6-dimethoxy-1,3,5-triazine). Originally reported as a shelf-stable intrinsically safe and non-explosive material, an academic and industrial team from Imperial College London and a process chemistry group at GSK subsequently found that the compound underwent highly exothermic decomposition comparable to tosyl azide.8 In fact ADT shows an exothermic decomposition with an initiation temperature of 159°C and an enthalpy of decomposition of -1135 J/g. The original paper failed to take into account that running DSC in an open crucible is not best practice (Figure 4). The fact that no material remained once the experiment had been run should also have been a hint that something else was going on.
Back to the case in point- Sperry’s team ran DSC on a sample of TMZ and found that rapid decomposition occurred at 170°C, releasing 1430 J/g of energy- with a maximum heat flow of 80 W/g. Somewhat lower than the 212°C melt/decomposition temperature reported by Hergenrother. TMZ is available from a range of suppliers and the team sourced samples from 5 different vendors. The supplied materials arrived in a range of colours (from red to orange to blue)- good for socks or T-shirts but rather alarming for a pure reagent (or an API in this case!). Reported purities were in the range 97% to >99%. DSC results of the various lots proved similar, however one lot showed a significantly lower decomposition onset temperature (108°C). None of the commercial batches were stable up to the reported melting point. Again a cautionary tale here too- trace impurities can lower the decomposition temperature and batch variation is a common cause of unpredicted runaway behaviour.
Its not just about the amount of energy that can be released, but how rapidly that energy is released and the increase in volume and pressure of gaseous decomposition products. Some compounds can explode, and many are sensitive to mechanical shock or friction. The ability to predict this type of behaviour from DSC data is possible using a mathematical relationship between the decomposition onset temperature and the amount of energy released. First described by Yoshida and later modified slightly by Pfizer (to reduce the possibility of false negatives), this calculation generally gives a good correlation with experimental results.9 Calculations based on the Pfizer-modified equation showed all five batches of Temozolomide were potentially shock sensitive and capable of explosive propagation.
Quite rightly these results triggered a cascade of further testing at Vertex. Most organisations have a fairly standard work-flow of extended safety experiments to help paint what can be quite a complex picture. Unfortunately, no grand unifying technique can give you all the answers you require. Impact testing showed the compound does indeed start smoking if you hit it. And you don’t need to exert much force- 9.8J/g. This is not too far off the kind of energy involved in dropping a flask.
ARC (accelerated rate calorimetry) was also undertaken to give a more detailed picture of the decomposition event and associated pressure increase. Thus is a very useful technique for ‘abusing’ materials and mimicking what might happen under thermal runaway conditions. The thermal onset detected in the ARC experiment was 145 °C and resulted in a pressure rise rate of >1114 psi/min. It’s not unusual for the decomposition onset temperature measured by ARC to be lower than that obtained in a DSC experiment. The former tends to be the more accurate (realistic) number. Further UN tests (relating to standardised tests for classification of materials ostensibly for shipping dangerous goods) revealed that Temozolomide should provisionally classified as a Class 1 explosive material.10 A turn-up for the books indeed.
To anyone involved in synthesis- particularly academic groups or inexperienced chemists in industry, I would say be very sceptical of safety data presented in the literature. Although papers are peer reviewed, it’s unlikely that the reviewers will insist on detailed (and potentially expensive) safety testing- even if the reported structures contains a red-flagged functional group. The authors (and reviewers) are very unlikely to have any background or experience in safety testing. The Vertex paper suggests that any published manuscripts claiming that a material is safe should include thermal stability data, including full details of how the tests were run. I completely agree.
Jeff Sperry’s team publish a great deal of pragmatic and useful information on safety testing- including highlights on social media. They are good advocates for process safety and raising awareness within the wider chemistry community.
Temozolomide remains a fascinating molecule and is unparalleled in its ability to treat some devastating cancers. Its use as reagent is somewhat more limited.
See time next time.
J
Scientific Update run a number of courses on process safety, including partnering with DEKRA- a world leader in process safety. For more information on in-house courses drop me an email ([email protected]) or for scheduled courses visit our website (www.scientificupdate.com).
References:
- Imidazotetrazines as weighable diazomethane surrogates for esterifications and cyclopropanations: P. Hergenrother et al, Chem. Int. Ed. 2019, 58, 2-8.
- Temozolomide – birth of a blockbuster: S. Sansom https://www.chemistryworld.com/features/temozolomide-birth-of-a-blockbuster/3004811.article
- Antitumor imidazotetrazines. 1. synthesis and chemistry of 8-carbamoyl-3-(2-chloroethyl)imidazo- [5,l-d]-l,2,3,5-tetrazin-4(3H)-one, a novel broad-spectrum antitumor agent: M. Stevens et al, J. Med. Chem. 1984, 27, 196−201.
- Recent enabling technologies for diazomethane generation and reactions: O. Kappe et al, Aldrichimica Acta 2016, 49, 57-66; Scalable on-demand production of purified diazomethane suitable for sensitive catalytic reactions: J. Sheeran et al, Process Res. Dev. 2021, 25, 522-528.
- Development of a continuous process for the industrial generation of diazomethane: L. Proctor et al, Org. Process. Res. Dev.2002, 6, 884–892.
- Importance of thermal stability data to avoid dangerous reagents: Temozolomide case study: J. Sperry et al, Org. Process Res. Dev. 2021, 25, 1690−1700.
- On the use of differential scanning calorimetry for thermal hazard assessment of new chemistry: avoiding explosive mistakes: J. Bull et al, Chem. Int. Ed. 2020, 59, 15798-15802.
- a) Intrinsically safe and shelf-stable diazo-transfer reagent for fast synthesis of diazo compounds, S. Xie et al, J. Org. Chem., 2018, 83, 10916-10921; Diaz0-transfer reagent 2-azido-4,6-diemethoxy-1,3,5-triazine displays highly exothermic decomposition comparable to tosyl azide: b) S.P. Green et al (GSK, imperial College), J. Org. Chem., 2019, 84, 5893-5898.
- Prediction of fire and explosion hazards of reactive chemicals. I. Estimation of explosive properties of self-reactive chemicals from SC-DSC data: T. Yoshida et al, Kogyo Kayaku 1987, 5, 311−316; Thermal stability assessment of peptide coupling reagents commonly used in pharmaceutical manufacturing. J. Sperry et al, Process Res. Dev. 2018, 22, 1262−1275.
- See https://www.hse.gov.uk/explosives/classification/un-test-results.htm








