Written by John Studley – May 7th, 2024.
As most process chemists know (but perhaps not so many discovery chemists) DMSO is nasty material. It undergoes explosive autocatalytic decomposition at or near its boiling point (189°C) generating non-condensable gases and concomitant extremely rapid pressure increases that can rupture a reactor vessel like a knife through butter. In terms of reaction kinetics, decomposition of the pure solvent at 195°C results in a 3ºC min-1 temperature rise and a pressure increase of 100 psi min-1 at maximum rate. As the temperature increases exponentially, following Arrhenius kinetics, the rate accelerates, and the pressure exceeds the safe operating pressure of the vessel and either triggers venting or ruptures the vessel. Pure DMSO releases 70 W/Kg. A mixture of DMSO and chloroform 640 W/Kg and DMSO in the presence of sodium hydroxide 2435 W/Kg!
To make matters worse the decomposition onset temperature is generally lower if anything is dissolved or in contact with it- reagents, catalysts, starting materials, flakes of rust from the vessel walls- you get the picture. Traces of HBr have been known to initiate a thermal runaway. Oxidants are to be avoided at all costs. There are plenty of papers that discuss this in more detail- I’ve listed some of the important ones in the references.1Any process using DMSO needs thorough safety testing or ideally re-engineering with another solvent. And if you’re thinking about another dipolar aprotic solvent they all have their issues. DMF in particular is another one to avoid if possible.2 Sulfolane you say?- in my experience it generally isn’t a good substitute.3 In many ways DMSO and DMF are more akin to reagents than inert, benign reaction solvents.1d,e
At our recent OPRD conference in San Diego Qiang Yang from Eli Lilly gave an interesting presentation on reaction safety evaluation.1a The conclusions were that if you heat DMSO your likely to run into trouble. During the question and answer session I innocently asked how DMSO was manufactured, ostensibly to figure out how the material was isolated, purified and handled on multi-thousand ton scale without inadvertently triggering a thermal runaway. Curiosity piqued I thought I would investigate the subject in more detail.1f
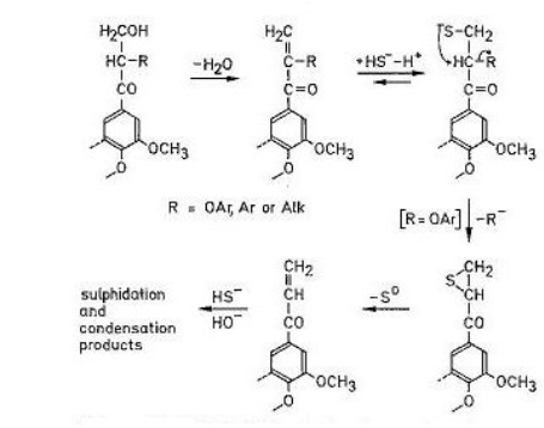
DMSO was discovered in the late 19th century as a minor by-product from the Kraft process, used in lignin degradation and wood pulping.4 Kraft comes from the German word “kraft”, meaning strength. Further research by a Russian chemist, Alexander Zaytsev, in 1867 showed that the Kraft process generates significant quantities of dimethyl sulfide and that oxidation of this material gave DMSO.5 The Kraft process is run on a mind-blowing scale. The largest plants produce in excess of 3,500 tons of pulp per day from wood chips in pressurised digesters- a process referred to as “cooking”- a term my fellow Breaking Bad fans will be familiar with. The digestion broth consists of a heated aqueous mixture of sodium hydroxide and sodium sulfide, known in the trade as “white liquor”. Sulfide has two benefits in kraft pulping: it promotes and accelerates the cleavage of ether linkages in phenolic units, assisting the break-up of the lignin macromolecule to smaller, more soluble units and it reduces the degree of undesirable condensation reactions. (Figure 1).6
Natural lignin comes from polymerisation of monolignols (p-coumaryl alcohol, coniferyl alcohol and sinapyl alcohol) which ultimately originate from processing of glucose through the Shikimic acid pathway (Figure 2).

Once the cooking time is up (2-3hrs at 170°C) the pulp is filtered. The unwanted components consist of a high pH, viscous black oil known as black liquor, Figure 3. Approximately 7 tons of black liquor are produced for every ton of pulp.

Where does the dimethyl sulfide come from? The black liquor contains all manner of volatile sulfur compounds, including MeSH, Me2S and MeSSMe. During Kraft pulping the aryl-methoxy groups in lignin are de-methylated by hydrosulfide anions generating methyl mercaptan anion that then goes on to cleave another methyl group generating dimethyl sulfide (Figure 4). Dimethyl disulfide is also formed by competing oxidation of the methyl mercaptan anion. During the cooking process elemental sulfur is also formed. This reacts with hydrosulfide to generate polysulfides. It’s a real witches brew. I imagine the smell.- well I can’t imagine it.6

How does DMSO fit into the equation? In the 1960’s the Gaylord Chemical Corporation developed a manufacturing facility in Bogalusa, Louisiana to make DMSO from dimethyl sulfide originating from black liquor generated in the Kraft process. A simplified “sulfur alkylation” is shown in Figure 5. DMS is volatile (b.p. 37°C) and easily purified by distillation. The oxidation process used to convert this material to DMSO is described below.

This process was run from the early 1960’s until 2010-the attraction was the use of a bio sustainable raw material.7
Not surprising there are multiple organisations globally that manufacture DMSO and productions costs are a major issue.
My assumption that pulp lignin depolymerisation is still used to make the DMS required for DMSO production might not be entirely correct. In 2010 Gaylord changed their process to a more cost-effective vapour-phase thioetherification using hydrogen sulfide and methanol over an aluminium oxide catalyst.8 I’m not entirely sure, but I imagine most manufacturers followed suite. However, a 2018 paper by Cheng et al published in ACS Sustainable Chem. Eng. still describes industrial manufacture of DMSO from lignin.9
Interestingly DMSO produced via the lignin process can be distinguished from material originating from the methanol/H2S process using mass spec. DMS from lignin contains higher levels of 14C.10
Methane thiol formation using syngas (CO/H2) or carbon disulfide hydrogenation has been reviewed.11
Obviously, we shouldn’t overlook the final step in the process- oxidation of the sulfur to sulfoxide. This is carried out using either oxygen gas or with nitrogen dioxide. Other oxidant we might use in the lab (peracids, metals/peroxides or biocatalysis) are too expensive to use at multi-hundred ton scale. Gaylord used dinitrogen tetroxide (NTO), which is formed as an equilibrium mixture with nitrogen dioxide.12 Its nasty stuff- used as rocket propellant in combination with hydrazine. In 1995 a railroad tanker at the Bogalusa factory containing N2O4 over pressurised and vented through a bursting disc. Large portions of the surrounding area were evacuated and various lawsuits were filed against Gaylord and their N2O4 supplier, Vicksburg chemical.
In terms of isolation of DMSO, it looks like its purified by low-temperature vacuum distillation. I’m sure there are plenty of checks and measures in place to prevent any possibility of an autocatalytic decomposition.
A few interesting facts to finish off. In September 2023 NASA announced detection of a potential signature of extra-terrestrial life in the atmosphere of a planet located 120 light years away from Earth (K2-18b). The James Web space telescope detected dimethyl sulfide in the planet’s atmosphere and concluded that this was a possible indication of life. Unless ET is cooking lignin it may a product of biochemical processing- a natural source of DMS on Earth.
Finally DMSO is used in several medical applications including topical pain relief, treatment of interstitial cystitis and in cryopreservation. Apparently some people believe that lignin- derived DMSO is more therapeutically active than petroleum-based DMSO. The homeopathic consort spouting their smoke and mirror pseudoscience again. I wonder if my reactions would have worked better in lignin derived DMSO…..somebody stop me.
See you next time.
References:
- a) Potential explosion hazards associated with autocatalytic thermal decomposition of Dimethyl sulfoxide and its mixtures: Q. Yang (Corteva) et al, Org. Process Res. Dev. 2020, 24, 916-939; b) Study on autocatalytic decomposition of dimethyl sulfoxide (DMSO): Y. Deguchi (Kaneka) et al, Org. Process Res. Dev. 2020, 24, 1614-1620; c) safe scale-up of pharmaceutical manufacturing processes with dimethyl sulfoxide (DMSO) as solvent and a reactant or a by-product Z. Wang et al (AbbVie), Process Res. Dev., 2014, 18, 1836-1842; d) Dimethyl sulfoxide as a synthon in organic chemistry J. Menesh et al, Synthesis, 2016, 48, 1421-1436; e) The applications of dimethyl sulfoxide as a reagent in organic synthesis: X. F. Wu et al, Adv. Synth. Cat. 2016, 358, 336-352; f) Sulfones and sulfoxides: K. M. Roy, Ullmann’s Encyclopaedia of Industrial Chemistry 2000, https://doi.org/10.1002/14356007.a25_487
- Potential safety hazards associated with using N,N-dimethylformamide in chemical reactions: Q. Yang et al, Process Res. Dev. 2020, 24, 1586–1601
- Sulfolane: A versatile dipolar aprotic solvent: U. Tilstam Process Res. Dev. 2012, 16, 1273–1278
- Pulp: M. Ragner et al, Ullmann’s Encyclopaedia of Industrial Chemistry 2014, https://doi.org/10.1002/14356007.a18_545.pub4
- History of DMSO: DMSO.org.uk
- Minimizing the sulphur content in Kraft lignin, Sara Svensson, 2008, http://mdh.diva-portal.org/smash/get/diva2:1676/FULLTEXT01.pdf
- Gaylord Chemical Corporation: https://www.google.co.uk/url?sa=t&source=web&rct=j&opi=89978449&url=https://en.wikipedia.org/wiki/Dimethyl_sulfoxide&ved=2ahUKEwjVo6yfkOCFAxWgQEEAHYECCAwQFnoECC4QAQ&usg=AOvVaw1a6ZYEEvfZKE0tS6bz1TXZ
- New method of dimethylsulfide synthesis: A. Mashkina et al, J. Org. Chem. 2011, 47, 678–681
- Dimethyl sulfoxide assisted ionic liquid pre-treatment of switchgrass for isoprenol production: G. Cheng et al, ACS Sustainable Chem. Eng. 2018, 6, 4354–4361
- Advances in resources recovery of H2S: a review of desulfurization processes and catalysts: L. Shen et al, ACS Catal.2023, 13, 11723–11752
- Methyl mercaptan production – catalysts and processes: V. Hulea et al, Sci. Technol 2023, 13, 3762-3778
- Process for the oxidation of organic sulfides: US2925442 (Gaylord)








