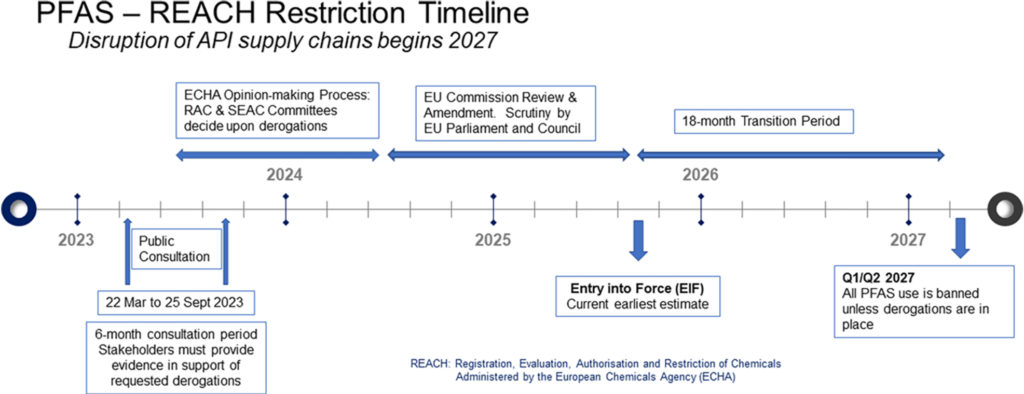
In December 2023 Nick Tyrell from ALMAC gave an interesting and timely presentation at our Winer Process Chemistry Conference in Liverpool, UK on the proposed EU ban of all per– and poly-fluorinated materials, including polymers and the fluorinating reagents we use routinely to introduce fluorine or fluorine-containing functional groups into complex molecules. The details of the proposed changes were recently highlighted in the Organic Process Research and Development journal.1 These changes could potentially have a profound impact on the chemical industry (Figure 1) including serious disruption to API supply chains beginning in 2027.2 The proposal stems from emerging concerns about the toxicity and environmental persistence of these so called “forever chemicals”.3 The Royal Society of Chemistry in the UK has also published a policy position on the topic.4a An outright ban would potentially have catastrophic consequences. A more pragmatic approach would be tighter controls and “essential use” arguments, similar to those used for CFC’s after the signing of the Motreal Protocol in September 1987.5 The US Environmental Protection Agency are also looking closely at PFAS,4b as are the Agency for Toxic Substances and Disease Registry (ATSDR).4c
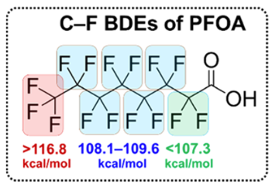
So what are Perfluorocarbons (PFC’s)? They are human-made chemicals that consist almost exclusively of carbon and fluorine. They are purely a product of human ingenuity- Nature is not at all interested in molecules of this type. The reason they are so pervasive stems from their exceptional chemical and thermal stability. They are also hydrophobic and solvent (oil) repellent- properties that make them attractive in many industries infra vide. These properties originate from the high C-F bond energies in per– and poly-fluorinated materials. Taking perfluorooctanoic acid (PFOA) as an example, it’s been estimated that the bond dissociation energies (BDEs) of the terminal −CF3 bonds in this molecule (117.8−123.4 kcal/mol) are higher than those of the −CF2− bonds (106.4−113.6 kcal/mol), with the CF2 adjacent to the carboxy- group having (not surprisingly) the lowest energy (Figure 2).6
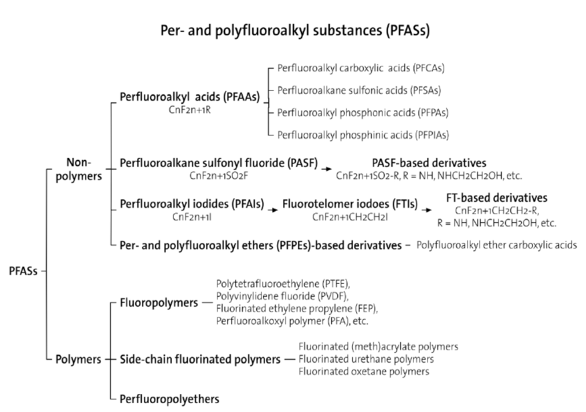
These unique characteristics have made them valuable in various industrial and consumer applications including the manufacturing of electronic devices and semiconductors, firefighting foams, and textiles/fabrics. Perfluorooctanoic acid (PFOA) and perfluorooctanesulfonic acid (PFOS), are used in the production of non-stick cookware and food packaging. More than 3000 per– and poly-fluoroalkyl substances (PFAS’s) are, or have at some stage been, available on the global chemicals market (Figure 3).7
Ironically, this exceptional chemical stability will also drive their inevitable legislative demise. PFCs are highly resistant to degradation in the environment- there are no natural enzymes that can break unactivated C-F bonds- leading to bioaccumulation and biomagnification in the food chain. PFCs have been detected in water bodies worldwide, including drinking water sources. There is a growing focus on removing parts-per-billion to parts-per-trillion levels of PFAS contamination from drinking water supplies. Detection at these very low levels can be analytically challenging.8 The health implications are not yet fully understood, however several studies have pointed toward developmental issues, liver damage, and disruption of the endocrine system.9
Despite biological systems for direct C-F bond cleavage being a non-starter, most PFAS’s materials have additional functionality adjacent to a fluorinated carbon that can act as a metabolic activator (Figure 4).6 In fully fluorinated molecules such as perfluorooctane, enzymatic hydrolysis is not observed. Reduction of the C–F bond requires electrochemical potentials below −2000 mV. The lower limit reduction potential for biological enzyme co-factors is around -600 mV. Whether enzyme-facilitated hydrolysis of perfluorinated molecules will ever be feasible remains to be seen.
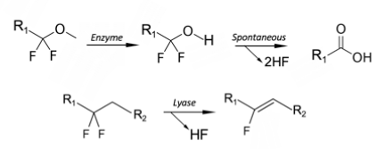
If the C-F bond is adjacent to a carbonyl, as is the case with naturally occurring fluoroacetate, direct C-F bond metabolism is facile and driven by a family of wildtype fluoroacetate dehalogenase enzymes.10a Fluoroacetate is one of the few naturally occurring organofluorine compounds, and is produced by as many as 40 different plants in Australia, Brazil, and Africa.11 It’s extremely toxic to animals (and humans), and is used as a defence mechanism to prevent the former (and possibly the latter) from eating them. It’s a Krebs cycle poison- inhibiting aconitase in the tricarboxylic acid cycle via “lethal” synthesis of an isomer of fluorocitrate.10b
If nature is struggling to break these compounds down, what are human beings doing to clear up their mess? A number of groups have reported the development of methods for degradation and mineralization of PFAS-type molecules including thermal destruction (which requires temperatures in excess of 1000°C),12 chemical oxidation with oxidants including ozone, hydrogen peroxide, persulfate, advanced systems such as Fenton’s reagent/ UV/H2O2 and electrochemical processing, all of which generate reactive intermediates that fragment the PFAS carbon chains. Photochemical destruction, using high energy UV in the presence of metals such as Titanium (oxide) has also been successful.13 The precise mechanisms for these processes are not always fully understood but they all aim to push the materials back through the fluorine cycle.
Chemical degradation of one of the particularly bad actors in this story- perfluorooctanoic acid (PFOA)- is an energetic process involving decarboxylation in an aprotic solvent (DMSO) (producing a C-H “handle”) followed by treating with a large excess of NaOH at 40°C (Figure 5).14 Deprotonation generates a reactive perfluoroanion that undergoes a cascade of fragmentation reactions, ultimately delivering fluoride and low molecular weight carbon fragments (Figure 5).
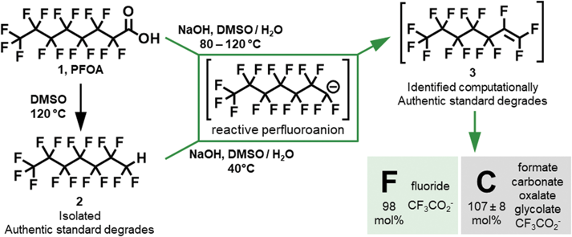
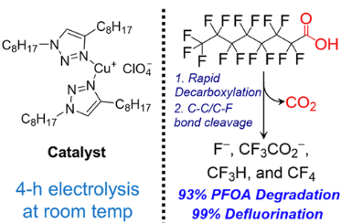
Jiang et al recently reported an electrochemical degradation of perfluorooctanoic acid using a copper catalyst, which again produces low molecular weight fluorinated “fragments” (F–,CF3CO2–, CF3H and CF4, Figure 6).15 The latter (CHF3 and CF4) are very potent greenhouse gasses. Playing whack-a-mole with Nature- a game we will almost certainly lose.
To round things off, another challenge. How do we dispose of PTFE((poly)tetrafluoroethylene)? Is all the PTFE we’ve ever made accumulating in the four corners of the Earth like Lego bricks? It’s estimated that 100,000 tons are produced every year! PTFE is manufactured (if we fully regress) from CaF2, which is converted to HF then on to the CF2=CF2 monomer (via CHClF2).16 Radical polymerisation produces the totally inert white-stuff we can’t live without. Again, what makes it special makes it dangerous (from an environmental perspective). All is not as bad as it seems. Vacuum pyrolysis at around 500°C rapidly converts it back to monomers (with a half-life of 30 minutes) via a depolymerisation process. It is, however, a very energy-intensive process. What would be very interesting would be to convert PTFE into a fluorinating reagent that could be used directly to fluorinate another molecule. A paper by Mark Crimmin and his team at Imperial College in London describes precisely that (Figure 7).17a
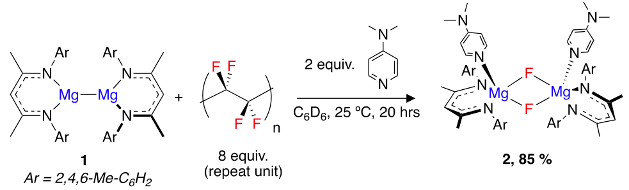
The reported procedure seems relatively straightforward. Powdered PTFE (1 μm particle size, Mn = 106−107, 8 equiv. of repeat units) is treated with a magnesium-based reducing agent (1), and DMAP, in benzene (!) and stirred for 16hr at 25°C. Preparation of the magnesium complex on 10g scale is described in a paper by Jones et al.17b The dimeric fluoride complex (2) was isolated as a dark grey polymer solid in 85% yield (based on (1) as the limiting agent, using 1,2-difluorobenzene as an internal standard in an NMR assay). No other fluorinated materials were observed.
(2) was found to be soluble in hydrocarbons and could be used as an inorganic nucleophilic fluoride source. For example reaction with BF3/nBu4NCl generated the corresponding tetrafluoroborate anion BF4– [nBu4N+].
There we have it. Ironically, the C&EN molecule of the year in 2022 was a perfluorcarbon- perfluorocubane. I wrote a blog article on this very interesting molecule last year.18a And in yet another twist of fate 1-(Perfluorohexyl)octane was approved by the FDA for treatment of dry eye disease in 2023. I wrote a blog article on that too.18b
I’m off to cook my dinner in my Teflon-coated pan.
See you next time.
References:
- A proposal that would ban manufacture, supply, and use of all fluoropolymers and most fluorinated reagents within the entire EU: N. Tyrrell. Process Res. Dev. 2023, 27, 1422-1426
- ECHA receives more than 5,600 comments on PFAS restriction proposal: https://echa.europa.eu/-/echa-receives-5-600-comments-on-pfas-restriction-proposal
- The wide presence of fluorinated compounds in common chemical products and the environment: a review: W. Zang et al, Sci. Pollut. Res. 2023, 30, 108393–108410
- a) Risk-based regulation for per- and poly-fluoroalkyl substances (PFAS) https://www.rsc.org/globalassets/22-new-perspectives/sustainability/a-chemicals-strategy-for-a-sustainable-chemicals-revolution/pfas-policy-position-dec-2021.pdf; b) https://www.epa.gov/pfas ; c) https://www.atsdr.cdc.gov/pfas/index.html
- a) The concept of essential use for determining when uses of PFAS’s can be phased out: I. Cousins et al, Sci. Processes Impacts, 2019, 21, 1803-1815; b) The importance of the Motreal Protocol in protecting climate: G. Velders et al, PNAS 2007, 104, 4814-4819
- Strategies for the biodegradation of polyfluorinated compounds: L. P. Wackett Microorganisms 2022, 10, 1664
- A never-ending story of per- and polyfluoroalkyl substances (PFASs): Z. Wang et al, Sci. Technol. 2017, 51, 2508–2518
- Polyfluoroalkyl substances (PFASs) detection via carbon dots: A review: L. P. Da silva et al, Sustain. Chem. 2023, 4, 339–362
- The discovery and analysis of PFAS (‘forever chemicals’) in human blood and biological materials: A. Travis, Substantia 2024
- a) Enzymatic fluorination and biotechnological developments of the fluorinase: D. O’Hagan et al, Chem. Rev. 2015, 115, 634-649; b) Toxicology of fluoroacetate: a review, with possible directions for therapy research: R Jenkins et al, J. Appl. Toxicol. 2006, 26, 148-161
- Fluoroacetate in plants- a review of its distribution, toxicity to livestock and microbial detoxification: L. Leong et al, J. Animal Sci. Biotechnol. 2017, 8, 55
- Thermal decomposition of two gaseous perfluorocarboxylic acids: products and mechanisms: D. Hanigan et al, Sci. Technol. 2023, 57, 15, 6179–6187
- A review of PFAS destruction technologies: J. Meegoda et al, I J. Environ. Res. Public Health 2022, 19, 16397
- Low-temperature mineralization of perfluorocarboxylic acids: B. Trang et al, Science 2022, 377, 839-845
- Molecular cu electrocatalyst escalates ambient perfluorooctanoic acid degradation: J. Jiang et al, Am. Chem. Soc. 2023, 145, 27390–27396
- Polyltetrafluoroethylene: synthesis and characterisation of the original extreme polymer: B. Ameduri et al, Chem. Rev. 2019, 119, 1763-1805
- a) Room temperature defluorination of poly(tetrafluoroethylene) by a magnesium reagent: M. Crimmin et al, J. Am. Chem. Soc. 2023, 145, 10486-10490; b) Dimeric magnesium(I) b-diketiminates: a new class of quasi-universal reducing agents: C. Jones Rev. Chem. 2017, 1, 0059
- a) Perfected cubane: J. Studley blog post Sept. 2022, https://www.scientificupdate.com/process-chemistry-articles/perfected-cubane/ ; b) Not a dry eye in the house: J’ Studley blog post July 2023, https://www.scientificupdate.com/process-chemistry-articles/not-a-dry-eye-in-the-house/








