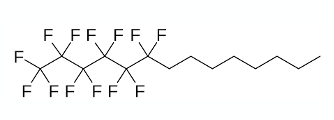
Yesterday I glanced through the list of drugs approved by the FDA in Q2 2023. One in particular, Miebo,1 -developed by Bausch and Lomb- made me sit up and stare. The compound is a partially fluorinated alkane (1-(Perfluorohexyl)octane, Figure 1) and is administered directly into the eye as a neat, anhydrous liquid to treat dry eye disease. It works by stabilizing the tear film on the surface of the eye, stopping the tears from evaporating. The idea of putting something like that in my eyes horrified me. Not one to resist a challenge, and suffering from dry eyes myself, I thought I’d give them a try. Its available over the counter in Europe, or in my case via amazon. Other suppliers are available. I have to report that I didn’t experience any of the pain and discomfort I expected, and my eyes did feel a little better (most likely a placebo component here- the drops were extortionately expensive so they had to be doing something right?). Figure 2 shows me and my £6.50/ml purchase.
The chemist in me soon started thinking about the molecule itself. How it’s made and purified, and, oddly, whether it would be a good solvent for chemical reactions! If you’ve read my blog articles in the past you will be aware of my fondness of fluorinated alkanes.2 Trying to find a literature reference to a process for making the compound proved to be a lot more difficult than expected. As my searching became deeper- with very few leads- I came across a patent filed by Novaliq, a pharmaceutical company focusing (no pun intended) on the development and commercialization of ocular therapies and water-free eyedrop technologies.3 In fact a patent examiners report-listing relevant prior art- was the only place I could find solace in peer-reviewed scientific literature.4
Before I discuss my findings, lets address the elephant in the room. This is the most heavily fluorinated “drug” approved for a long while- maybe even all time. And as many of you are probably aware there is currently an ongoing public consultation in the EU on a proposed restriction that would ban all PFAS (Per- and polyfluoroalkyl substances and materials), including vital materials such as TFA and fluoro-polymers, due to environmental and public health concerns. I would imagine a partially perfluoroalkayated material used to treat a disease would be exempt, however the raw materials for its production might have eventually very tight restrictions or may even be phased out. These restrictions have serious long-term consequences for the fine chemical and pharma industries. Despite this, perfluorocarbons are used as commodity fluorinated precursors in very large volume (>100’000 to/a).6
The synthetic approach to semi-perfluorinated alkenes such as Miebo begins with an iodofluoroalkane. This family of compounds undergo radical coupling reactions with fluoro-olefins to generate chain-extended perfluoroiodo building blocks. Industrially, tetrafluoroethylene undergoes oligomerisation (or telomerization to give it its proper term) via free-radical initiation using heat, light or a radical initiator such as AIBN (Figure 3).7
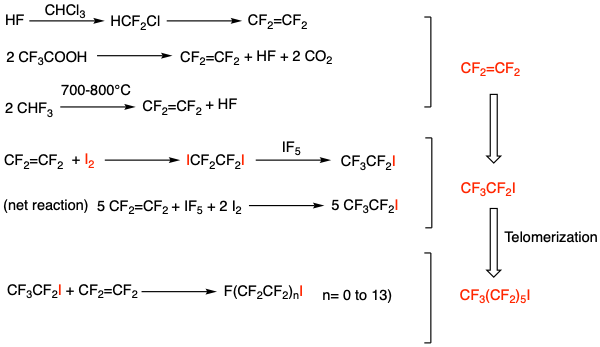
During the telomerization processes Iodine pentafluoride is reacted with tetrafluoroethylene to produce a fluorinated alkyl iodide with linear, even numbered alkyl chains, known as a fluorotelomers. Reaction of tetrafluoroethylene with iodine, followed by treatment with iodine pentafluoride (IF5) is used industrially to make pentafluoroiodoethane (CF3CF2I), a key intermediate for the synthesis of our 6-membered perfluorinated chain. Further 2-carbon telomerization gives our hexyl-based fragment required for manufacture of Miebo. Iodofluoroalkanes can also be made by heating perfluorocarboxylic acid silver salts with iodine or the sodium salts with iodine in DMF.8 Usually the process is carried out the other way round- making perfluorocarboxylic acids from perfluoroalkyl iodides.
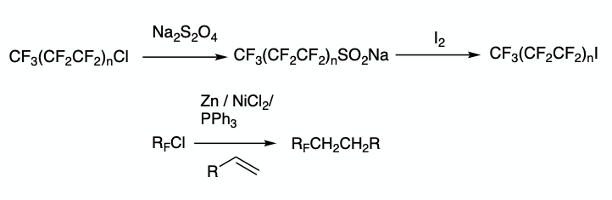
Perfluoroalkyl iodides have also been made using perfluoroalkyl chlorides via a modified sulfinatodehalogenation process and by nickel(0) catalysed processes (Figure 4).9
Surprisingly Iododfluoroalkanes are not alkylating agents, however they do form useful organometallic intermediates such as Grignard reagents.10 Our 1-iodoperfluoro-n-hexane, as with its lower and higher-chain analogues, is fairly volatile (b.p. 117°C, m.p. -45°C) so can be separated by fractional distillation. Are these intermediate PGI’s? I’m guessing not.
Regarding the two key raw material- Tetrafluoroethylene (gas) is made industrially using several different methods including pyrolysis of fluoroform and pyrolysis of chlorodifluromethane. It can also be generated by decomposition of TFA (Figure 2).6b,11 In the lab it can be made by pyrolysis of pentafluoropropionate salts.12b
It has a high degree of thermal instability and can undergo rapid polymerisation with very little persuasion. Adiabatic compression is sufficient to trigger an explosion. The stabilised material (using a-pinene or dipentene) is much safer to handle and transport. Tetrafluoroethylene’s main use is in the preparation of PTFE. A recent review by Ogoshi et al describes the application of tetrafluoroethylene as an industrial organofluorine feedstock.12
Iodine pentafluoride is a colourless, highly reactive liquid (melting point 9.4 °C, b.p. 98°C), made by ‘burning’ iodine in fluorine gas.13 Family in-fighting and the sprightly younger sibling wins hands down. Purification by distillation occurs without any decomposition at low temperature, however thermal activity commences at temperatures above 400 °C.
So once we have our perfluorinated intermediate we need to append the alkyl chain. There are a couple of different approaches described in the patent literature. The first is a radical coupling between the perfluoroiodo intermediate and an electron rich olefin using a radical initiator such as AIBN or benzoyl peroxide in the presence of a metal.7a A light-driven process has also been developed for similar transformations by the Noel group.14 Figure 5 shows an example using catalytic titanium (0) (generated in situ from TiCl4 and Zn) as a radical initiator.15 Reaction of 1-iodoperfluorohexane with 1-octene gives an intermediate chain-extended iodide that can be reduced with Zn/AcOH in a separate step, or as a telescoped on-pot procedure (Figure 5). An Iron bromide derived catalyst has been used in a similar fashion.15b
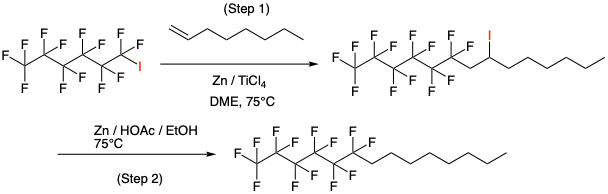
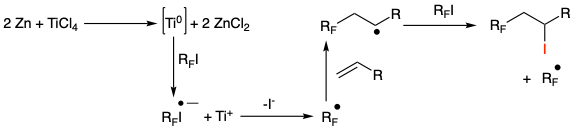
The proposed mechanism is believed to involve in situ generation of Ti(0) from reaction of TiCl4 with zinc, and single electron transfer with the iodide to give the radical anion (Figure 6). This is confirmed by reaction inhibition upon addition of a radical scavenger (p-dinitrobenzene).
Most of the patents in this area of chemistry focus on the novelty of methods for the reductive dehalogenation step. Urushibara metal catalysts derived from non-precious metals such as nickel, cobalt, iron or copper work very effectively.16 These catalysts are prepared by precipitation of the metal as a finely divided powder from an aqueous solution of a suitable salt-form using another metal with a greater ionization potential (e.g. zinc). In a typical procedure 10% NaOH solution is added to the powdered metal together with powdered zinc followed by heating at 50°C for a period of time. The resulting basic catalyst is used as a caltalyst in the alkyliodide reduction at 10 mol% together with stochiometric sodium borohydride. Reducing power is all about particle size and surface area.
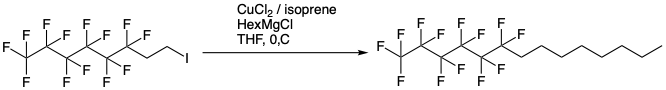
Yet another parented dehalogenation procedure involves formation of a Grignard reagent by an exchange process with iPrMgCl and copper-catalysed coupling of the appropriate alkyl halide (Figure 7).16b 1,1,2,2-tetrahydrofluoroalkyl iodide is made using a similar radical-driven process to that described above using ethene as the olefin component.17
So there we have it. An unusual structure for a medicine- especially one administered neat to your eyes. I have to report my eyes are no longer as dry as my sense of humour.
See you next time.
References:
- Dry eye paradise: FDA green-lights Miebo as first and only eye drop approved for the condition: D. Hutton, Ophthalmology Times, 46, June 2023; The history of FDA approval: https://www.drugs.com/history/miebo.html
- Perfected cubane: J. Studley blog article September 2022: https://www.scientificupdate.com/process-chemistry-articles/perfected-cubane/
- Semifluorinated compounds for ophthalmic administration: Novaliq Gmbh, WO2017055453A1
- International search report PCT/EP2018/061473
- Per- and polyfluoroalkyl substances in the environment: W. M. Henderson et al, Science 2022, https://doi.org/10.1126/science.abg9065
- a) Recent developments and aspects of industrial fluoroalkylation: M. Beller et al, CHIMIA 2021, 75, 923-935; b) Fluorinated compounds, organic, G. Siegemund et al, Ullmann’s Encyclopaedia of Industrial Chemistry 2000, https://doi.org/10.1002/14356007.a11_349
- a) Telomerisation reactions of fluorinated alkenes: B. Ameduri et al, Top. Cur. Chem. 1997, 192, 165-233; b) Chemistry, physical chemistry, and uses of molecular fluorocarbon-hydrocarbon diblocks, triblocks, and related compounds- unique “apolar” components for self-assembled colloid and interface engineering: M. P. Krafft et al, Chem. Rev. 2009, 109, 1714-1792
- Syntheses utilizing n-perfluoroalkyl iodides [RFI, CnF2n+1-I] 2000–2010: Buck et al, J. Fluorine Chem. 2012, 138, 3-23
- a) Practical and efficient synthesis of perfluoroalkyl iodides from perfluoroalkyl chlorides via modified sulfinatodehalogenation: Q, Y, Chen et al, J. Fluorine Chem. 2007, 128, 1187-1190; b) Nickel(0)-catalysed fluoroalkylation of alkenes, alkynes, and aromatics with perfluoroalkyl chlorides: X. T. Huang et al, Org. Chem. 2001, 66, 4651–4656
- For example: Synthesis of fluorinated amphiphiles by the reaction of protected hydroxy carbaldehyde with perfluorinated organomagnesium compounds: J. Kvicala et al, J. Fluorine Chem. 2002, 113, 195-200
- a) Method for the preparation of tetrafluoroethylene: DuPont US3081245; b) Production of fluorinated compounds: Pennsalt Chem US3009966;c) Pyrolysis of chlorofluoroalkanes: DuPont US2551573
- a)Transformation of tetrafluoroethylene using transition-metal complexes: R. Doi et al, Synthesis 2023, 55, 857-867; b) preparation of tetrafluoroethylene from the pyrolysis of pentafluoropropionate salts: J. Thrasher et al, J. Fluorine Chem. 2017, 196, 107-116; c) Advances in the synthesis and application of tetrafluoroethylene- and 1,1,2,2-tetrafluoroethyl-containing compounds: P. Beier et al, Chem. Eur. J. 2018, 27-28, 3554-3593
- Iodine pentafluoride (IF5) https://www.solvay.com/en/brands/iodine-pentafluoride-if5; Halogen fluorides: W. Bailey et al, https://doi.org/10.1002/0471238961.0801121502010912.a01.pub2
- For example see visible light-induced trifluoromethylation and perfluoroalkylation of cysteine residues in batch and continuous flow: T. Noel et al, J. Org. Chem. 2016, 81, 7301-7307
- a) Titanium-catalysed addition of perfluoroalkyl iodides to alkenes: D. Burton et al, J. Fluorine Chem. 1995, 70, 135-140; b) An optimized condition for practical and scalable hydrodeiodination of perfluoroalkyl iodides: C. Liu et al, J. Fluorine Chem. 2016, 184, 45-49
- a) The Urushibara catalysts: https://en.wikipedia.org › wiki › Urushibara_nickel; b) Process for the production of semifluorinated alkanes: Novaliq, WO2018/202835 A1; c) Process for the preparation of semifluorinated alkanes using Grignard reagents: Novaliq, 2018/228975A1
- Crosslinking of fluoroelastomers by “click” azide–nitrile cycloaddition: B. Ameduri et al, Polymer Sci., Part A: Polymer Chemistry 2015, 53, 1171-1173








