In the spring of 1940, John Archibald Wheeler, eminent theoretical physicist, called up his then PhD student, Richard Feynman (generally regarded as the most famous modern-age physicist) and said “Feynman- perhaps there’s only one electron in the Universe” a single entity moving backwards and forwards in time. If true it would be one hard working particle.1 Today that electron finds itself incarcerated in a fluorinated cube – and at the time of writing the world is still turning.
First a potted history of the magic cube. Cubane is a fascinating molecule (Figure 1). First synthesised in 1964 by Eaton and Cole, this highly strained hydrocarbon- despite its unusual physical properties- remained something of a chemical oddity, residing largely in theoretical chemistry publications.2 The molecule isthermodynamically unstable- the geometrical arrangement of the carbon atoms some way off tetrahedral, however it shows very high kinetic stability. If it’s heated nothing much happens below 200°C (!) suggesting there are no facile decomposition pathways.3 The fully nitrated analogue (octanitrocubane), however, is a very different storey. This material is a shock-insensitive high explosive.4 Theoretically cubane derivatives are energy storage units- ready to spring open and release all that pent-up tension.
The combination of novel structural architecture, low toxicity and kinetic stability has resulted in cubane analogues being prepared and screened by medicinal chemists and drug hunters as a phenyl ring isosteres.5 Cubane represents a somewhat unique template for functionalisation and positioning of appended substituents to make key interactions with biological targets. I’m sure many compounds have been made and screened, however there are currently no cubane-containing molecules in the clinic, something that may change over time.
Dimethyl 1,4-cubanedicarboxylate, prepared in 8 steps from cyclopentadienone ethylene ketal on kilogram scale by Tsanaktsidis et al, is the key building block for cubane chemistry (Figure 2).6 The procedure they adopted was based on a modification of the method reported by Chapman et al in 1970.7
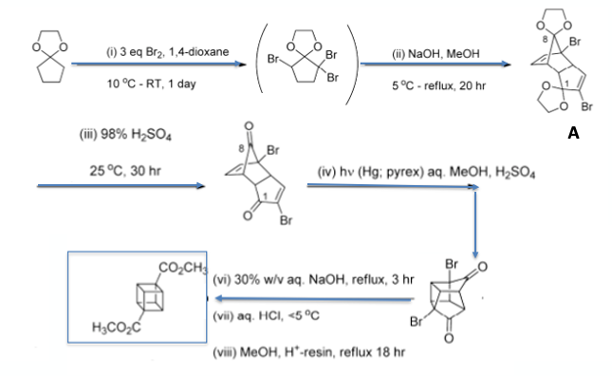
Mechanistically the sequence is interesting. Bromination of cyclopentanone ethylene ketal and subsequent dehydrobromination gives a very reactive intermediate- 2-bromocyclopentadienone ethylene ketal- that doesn’t hang around very long. It undergoes a stereoselective Diels-Alder dimerization to give the bis-ketal intermediate (A). Deprotection gives the bis-ketone. Photochemical [2π +2π] ene-enone photocyclization generates the cage dione. Treatment with sodium hydroxide promotes double Favorskii ring contraction giving the cubane framework. Linclau et al have developed a continuous photochemical synthesis of the dicarboxylate.6b The carboxylic acid provides a synthetic handle for elaboration of the cubane hydrocarbon via functional group interconversion and decarboxylation processes. Electrochemical conversion of cubane carboxylic acids to alkoxy cubanes using a Hofer-Moest reaction has recently been published by Brown et al.8
I guess it was inevitable that someone would eventually prepare fully fluorinated cubane (Figure 3). In my early industrial chemistry career, at ISC chemicals in Avonmouth (Bristol), I spent many hours working on the synthesis of fully fluorinated (perfluorinated) hydrocarbons-the so called ‘Flutec’ liquid fluorocarbons. These molecules were made via the Fowler process- a two- step process involving fluorination of CoF2 (obtained by reaction of Co(II) with HF) to CoF3 with F2 gas, followed by continuous high temperature fluorination of a vaporised hydrocarbon feedstock over the cobalt catalyst bed.9a The CoF2 generated by the redox process could then be converted back to CoF3. This process has its origins in manufacture of volatile fluorocarbons for the Manhattan project.9b
These inert perfluorinated molecules have very interesting physical properties, with application in advanced materials research and medicine. Try a google search on fluorocarbon blood substitutes.9c
Back to Perfluorocubane. Akiyama et al recently reported the synthesis and characterisation of this unlikely looking molecule in Science.10 In terms of its properties, the perfluorocarbon has a unique ability to kidnap an electron and form a radical anion, with the lonely electron incarcerated in a cubane cage.
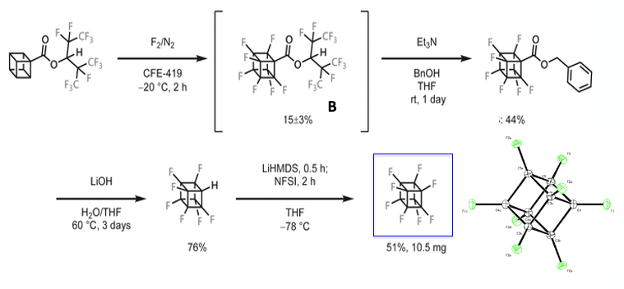
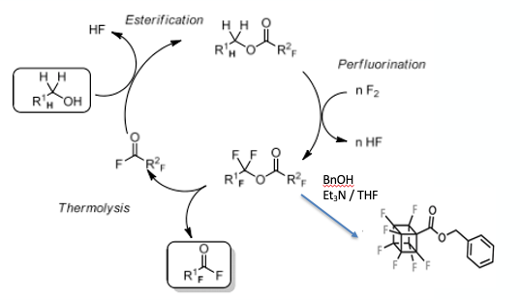
First to the synthesis. The obvious approach is direct fluorination of the cubane molecule itself. This has been attempted by others, however the strained ring system breaks open relatively easily. Akiyama’s team adopted the “PERFECT” (PERFluorination of an Esterified Compound then Thermolysis) method, reported by Okazoe (a co-author of the Science paper) and developed in 2015 by the Asahi Glass Co., Ltd, for manufacture of fluorinated materials (Figure 3) .11 This method relies on direct fluorination of a partially fluorinated ester (hexafluoroisopropyl in this case) in a fluorous ether solvent (1,2-dichloro- 3-(2-chloro-1,1,2,2-tetrafluoroethoxy)-1,1,2,3,3- pentafluoropropane ,CFE-419) – a method shown to inhibit C-C bond cleavage (Figure 4). Treatment of the cubanecarboxylate ester with fluorine gas at low temperature gave a low yield of the fully fluorinated intermediate (B, 15%, Figure 3). The intermediate was then telescoped into a transesterification reaction with benzyl alcohol. Hydrolysis and decarboxylation gave the penultimate mono-hydro perfluorocubane. The remaining hydrogen atom succumbed by anion formation (with LiHMDS, -78°C ) and fluorination with NFSI (N-fluorobenzenesulfonamide), again in modest yield (51%).
Single crystal X-ray analysis confirmed identical C-C bond lengths (1.570Å), indicative of a perfect cube, confirming predictions by high-level DFT calculations.
In 2008, Irikura predicted that a caged perfluorocarbons would have high electron afinities.12 The theory was that carbon-fluorine antibonding (s*) orbitals in a caged structure would face toward the centre of the cage and, depending on the cavity size, the s* orbitals will overlap in a process known as sigma stellation.12 An added electron would then quite happily occupy this new hybrid molecular orbital forming a radical anion.
The structure of the radical anion of perfluorocubane, resulting from introduction of an electron into the LUMO orbital derived from the eight C–F * orbitals, was confirmed by matrix-isolated electron spin resonance (ESR) spectroscopy in combination with low-temperature gamma-ray radiolysis. The upshot of a very detailed investigation was that spin density is mainly distributed inside the cubane cage.10
I guess the next question is what can you do with it? It’s probably not the best time to be serving up a new perfluorocarbon. These compounds can have a severe environmental impact due to persistence and accumulation in the biosphere- the so called “forever chemicals”.13 In fairness perfluorcubane is unlikely to become anything more than a chemical curiosity in the short to medium term. We won’t be seeing hundreds of grams appearing overnight. Trapping an electron might introduce some interesting redox behaviour. The fact that the charge is in the centre of a cage is intriguing- most electron acceptors locate additional electrons on their surface. In addition to possible application is advanced materials (semiconductors and organic conducting compounds etc.) an electron transporter might prove interesting in photoredox or electrochemistry. Perfluorocubane and other fluorinated cage structures could promote some interesting chemistry. Let’s roll the dice and see what happens…
See you next time.
References:
- https://en.m.wikipedia.org/wiki/One-electron_universe
- Cubanes: starting materials for the chemistry of the 1990s and the new century, P. Eaton, Chem. Int. Ed. 1992, 31, 1421-1436.
- Cubane: 50 years later, R. Priefer et al, Chem. Rev. 2014, 115, 14, 6719–674
- Hepta- and octanitrocubanes, P. Eaton, Chem. Int. Ed. 2000, 39, 401-404
- a) Ubiquity of cubanes in bioinorganic relevant compounds, M. Zdilla et al, Co-ord. chem. Rev. 2022, 450, 214168; b) Cubanes in medicinal chemistry, M. Kassiou et al, Med. Chem.2019, 62, 1078–1095; c) Cubanes in medicinal chemistry: synthesis of functionalized building blocks, J. Wlochal et al, Org. Lett. 2014, 16, 4094–4097
- a) Pilot-scale production of dimethyl 1,4-cubanedicarboxylate, J. Tsanaktsidis et al, Org. Process Res. Dev. 2013, 17, 1503–1509; Decagram synthesis of dimethyl 1,4-cubanedicarboxylate using continuous-flow photochemistry; b) B. Linclau et al, Synthesis 2021, 53, 1307-1314
- Preparations and properties of caged polycyclic systems. 1. Pentacyclo[5.3.0.02,5.03,9.04,8]decane and pentacyclo[4.3.0.02,5.03,8.04,7]nonane derivatives N. Chapman et al, Org. Chem.1970, 35, 3860–3867
- Cubane electrochemistry: direct conversion of cubane carboxylic acids to alkoxy cubanes using the Hofer–Moest reaction under flow conditions, R. Brown et al, Chem. Eur. J. 2020, 26, 374-378
- a) Perfluoroalkanes, G. Sandford, Tetrahedron 2003, 59, 437-454; Fluorocarbons by fluorination of hydrocarbons with cobalt trifluoride, R. Benner et al, Eng. Chem. 1947, 39, 329–333; b) The Manhattan project, H. Goldwhite J. Fluor. Chem. 1986, 33, 109-132; c) Perfluorocarbons as blood substitutes, K. Waxman, Ann. Emerg. Med. 1986, 15, 1423-142
- Electron in a cube: synthesis and characterization of perfluorocubane as an electron acceptor, M. Akiyama et al, Science 2022, 377, 6607, https://www.science.org/doi/10.1126/science.abq0516;
- a) Development of the ‘‘PERFECT’’ direct fluorination method and its industrial applications, T. Okazoe Fluor. Chem. 2015, 174, 120-131; b) Application of liquid-phase direct fluorination: novel synthetic methods for a polyfluorinated coating material and a monomer of a perfluorinated polymer electrolyte membrane, T. Okazoe et al, Appl. Sci. 2012, 2, 327-341
- Sigma stellation: a design strategy for electron boxes, K. Irikura, Phys. Chem. A2008, 112, 983–988
- Per- and polyfluoroalkyl substances in the environment, W. M. Henderson et al, Science 2022, https://doi.org/10.1126/science.abg9065








