When one thinks about the chemistry of alkenes, oxidative cleavage via ozonolysis will be close to the top of the list. Certainly in the top 5. Discovered by the Swiss chemist Friedrich Schönbein in 1839, this reaction remains -both academically and industrially- a highly efficient, sustainable method for introducing oxygen into both simple feedstock materials and complex synthetic targets.1 Many of the safety challenges and control aspects of this process have been addressed by the implementation of continuous flow systems including generation of ozone gas and the safe processing of unstable peroxy- intermediates.2
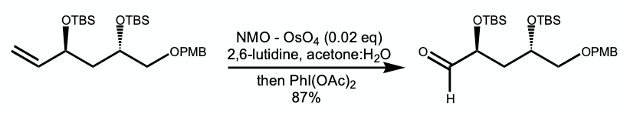
Addressing the issues associated with generation and handling of ozone gas, several metal-based methods have been reported, including Osmium/periodate (Lemieux Johnson oxidation) , Osmium/NMO/PhI(OAc)2(Nicolaou, Figure 1), Pd(OAc)2/O2 (Jiang) and methods combining oxygen gas with catalytic base metals such as cobalt and manganese.3 Kroutil et al have reported an enzymatic oxidative cleavage process, however the method is very substrate specific.4. These metal-based reactions tend to generate undesirable waste. In particular, osmium tetroxide is extremely toxic and difficult to handle.3b This has been offset to some extent by its unique ability to oxidise double bonds to diols and (under the right conditions) carbonyl derivatives. Incidentally the addition of 2,6-lutidine to the osmium/periodate oxidation suppress side reactions and dramatically improve the yield.5
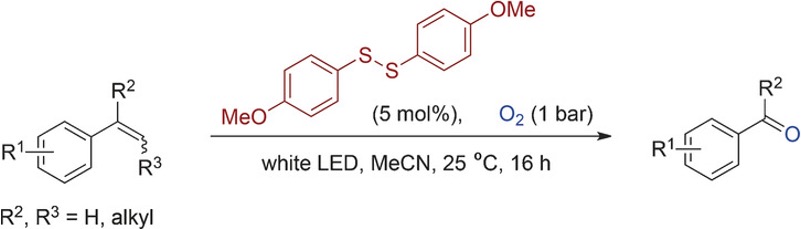
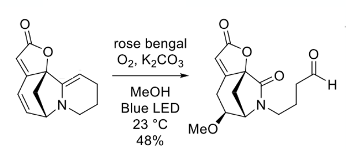
Photoirradiation methods have been used to activate oxygen via excitation of a suitable metal catalyst and generation of a more oxidizing high-valent metal–oxygen species. Coupling this redox process with alkene oxidation results in photo-mediated oxidative cleavage.3c Wang et al have developed a disulfide-catalysed visible-light-mediated oxidative cleavage of C=C bonds, again using molecular oxygen as oxidant (Figure 2).6 Han et al very recently described oxidative cleavage of an enamine with rose bengal in an oxygen atmosphere under blue LED irradiation (Figure 3). Cleavage of the C2−C3 bond gave a lactam- a key intermediate in his synthesis of the Securinega alkaloid flueggeacosine B.6b
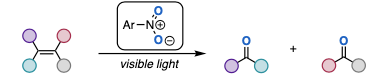
These methods are interesting- but what we really want is a method that avoids the use of oxygen gas, metals, and/or strong oxidants such as periodate. This is exactly what the Parasram group have given us. A recent publication in the J. Am. Chem. Soc. describes the photoinduced Anaerobic transfer of oxygen to alkenes using nitroaromatics as the source of oxygen.7 No exogenous oxidants required- just a lamp and a nitroaromatic compound (Figure 4).
The first step in ozonolysis of an alkene is a 1,3-dipolar cycloaddition reaction generating a primary ozonide. Nitro-groups can react in a similar way-the difference being that the reaction is thermodynamically unfavourable and requires a strained alkenes to lower the activation energy. Photochemical irradiation of a nitro group supplies the energy necessary to overcome this limitation, generating a higher energy (diradical) excited state that can trigger cycloaddition. The resulting dioxazolidine intermediate doesn’t hang around very long- fragmenting to the carbonyl products observed during standard ozonolysis chemistry (Figure 5). Oxidation of alkenes with nitrobenzene is not a new process- Buchi et al were looking at this in the mid 1950’s using (somewhat heavy handedly) UV radiation.8
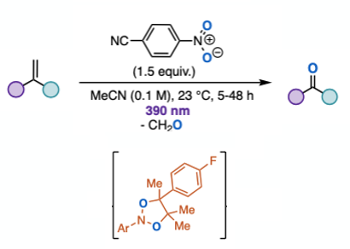
Milder photochemical activation using visible light significantly enhances the synthetic utility of the reaction. The hypothesis was that formation of a donor-acceptor (EDA) complex with an electron deficient nitroarene (4-cyanonitrobenzene) acceptor would facilitate the oxygen transfer event in an anaerobic cleavage of alkenes. The actual mechanism, based on experimental data, is highlighted below. Purple light (390nm) gave the best results in screening experiments using 4-fluorostyrene as substrate. In general styrenes worked well, however unactivated alkyl alkenes gave modest yields of carbonyl derivatives.7
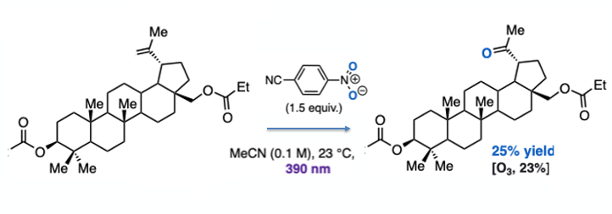
A notable example is the anaerobic cleavage of betulin- a pentacyclic triterpene natural product. Both the nitroarene process and standard ozonolysis gave identical yields (25% v’s 23% for standard ozonolysis, Figure 6).
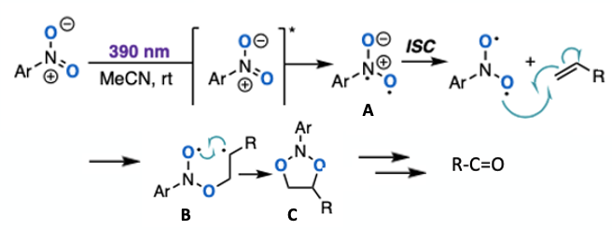
Not surprisingly a large section of the paper is dedicated to mechanistic studies. UV-visible spectroscopy, radical trapping and triplet quenching studies revealed that the transformation occurs via a solvent separated ion-pair complex. The Nitroarene is photoexcited by visible light (390 nm) to generate a dioxygen radical intermediate. The nitroarene excited state undergoes intersystem crossing (ISC) giving a diradical excited state (Figure 7, A) that engages in a radical cycloaddition event with the alkene to generate a short-lived biradical intermediate (B). Intramolecular radical ring closure leads to 1,3,2-dioxazolidine (C) that decomposes rapidly to furnish the carbonyl derivative (Figure 7). In situ photoNMR techniques enabled detection of a putative aryl 1,3,2-dioxazolidine intermediate.
At first glance this seems like a simple, practical method for oxidative cleavage of alkenes to generate versatile carbonyl intermediates. The procedure does not require employment of toxic metals or pose safety risks associated with the use of strong oxidants (including ozone). If you’re not too concerned about yield this is s a quick method for cleaving alkenes to carbonyl intermediate without the fuss of setting up ozone generators or handling Osmium.
See you next time.
References:
- Application of ozonolysis in organic synthesis- oxidation on demand: J. studley, 2022, https://www.scientificupdate.com/webinar_events/application-of-ozonolysis-in-organic-synthesis-oxidation-on-demand/20220405/
- a) An expedient procedure for the oxidative cleavage of olefinic bonds with phI(OAc)2, NMO, and catalytic OsO4, Lett., K. Nicolaou et al, 2010, 12, 1552–1555; b) Aerobic cleavage of alkenes and alkynes into carbonyl and carboxyl compounds, R. SanMartin et al, ACS Catal. 2017, 7, 3050–3060; b) Osmium (element 76), J. Studley 2019, https://www.scientificupdate.com/general/osmium-os-element-76/; c) Oxidative cleavage of alkenes by O2with a non-heme manganese catalyst, J. Xiao et al, J. Am. Chem. Soc., 2021, 143, 10005–10013
- Scale-up of ozonolysis using inherently safer technology in continuous flow under pressure: case study on β-pinene, Roth et al, Org. Process Res. Dev. 2021, 25, 1589−1597
- Oxidative alkene cleavage by chemical and enzymatic methods Synth. Catal.W. Kroutil et al, 2013, 355, 3321-3335
- Improved procedure for the oxidative cleavage of olefins by OsO4-NaIO4, Lett.2004, 6, 3217–3219
- a) Disulfide-catalyzed visible-light-mediated oxidative cleavage of c=c bonds and evidence of an olefin-disulfide charge-transfer complex, T. Noel et al, Chem. Int. Ed. 2017, 56, 832 –836; b) S. Han et al, J. Am. Chem. Soc. 2022, 144, 8932−8937
- Photoinduced oxygen transfer using nitroarenes for the anaerobic cleavage of alkenes; M. Parasram et al, Am. Chem. Soc. 2022, 144, 15437–15442
- Light catalyzed organic reactions. iv.1 the oxidation of olefins with nitrobenzene, G. Buchi et al, Am. Chem. Soc., 1956, 78, 689–690








