Osmium, a group 8 d-block transition metal discovered in 1803 by the English chemist Smithson Tennant, is the rarest of the stable elements. Concentrations in the Earth’s crust are around 50 parts per trillion. The metal is found in nature uncombinrd or alloyed with its neighbour, iridium, in the alloys iridosmine or osmiridium. Around 500Kg of osmium are produced industrially per year as a by-product of refining nickel and other more abundant platinum group metals. Canada is a main country of origin.1a

Figure 1: Osmium Crystals (CristalTech Sarl, Switzerland)
The name ‘osmium’ comes from the Greek word ‘osme’meaning smell. The metal itself is odourless- the pungent smell derives from it’s oxide, OsO4. The oxide has proved useful industrially, however it has significant toxicity. More about this later. The metal itself has limited direct industrial application. That said it forms alloys with other platinum group metals giving very hard wearing and chemically inert materials that are extremely resistant to compression.
The geologists have found a practical application: rhenium-osmium dating. This is a radiometric dating procedure based on the beta-decay of 187Re to 187Os and can be used to study mantle crust evolution and the age of ore deposits. The extremely long half-life (>40 billion years) can also be used to study the solidification of materials during Earth’s early history.1b
Historically osmium and its next-door neighbour iridium have been fighting over the title of densest element on Earth. The currently accepted values put osmium ahead by a hair’s breadth (22.587 g/cm3 at 20°C for Os v’s 22.562 g/cm3 for Ir). Having said that, osmium is the densest metal at all temperatures and ambient pressure(although there is an ambiguity below 150 K). However, at room temperature iridium becomes the densest metal above a pressure of 2.98 GPa, at which point the density is 22.750 g/cm3.2
Osmium has a very wide range of oxidation states- 11 in total ranging from -2 to +8. Although +8 is very high (OsO4 being an example), the high oxidation state trophy goes to its sparring partner iridium with an impressive +9.3
Osmium has no biological role but is present at a very low back-ground levels in the body. Medically, however, complexes of osmium have been found to inhibit the growth and proliferation of human cancer cells. Although this area is largely dominated by ruthenium several osmium complexes are comparable to the standard of care in this field, cisplatin, – at least in vitro.4 Many of these complexes are isostructural with the corresponding ruthenium derivatives. Mechanistically, depending on the metal ligand environment, a diverse range of modalities are possible including redox activation, DNA targeting or inhibition of protein kinases (Figure 2).5 An interesting paper just published by Fus et aldescribes replacement of the b- aromatic ring in 4-hydroxytamoxifen by metallocene’s (Fe, Ru and Os) to drive both hormone-dependant and independent antiproliferative activity in cancer cells.6a There is growing evidence that these so called metallocifens are prodrugs and are rapidly converted in vivo into electrophilic quinone-methides that react with thiol functionality viaa 1,8-Michael addition reaction.6b The team from Grenoble used the osmium complex to image and quantify intracellular distribution in triple negative breast cancer cells and used this data to propose a comprehensive mode of action for this promising new class of metallodrugs.6c
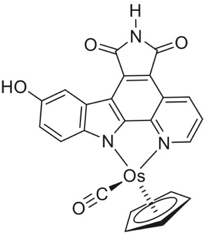
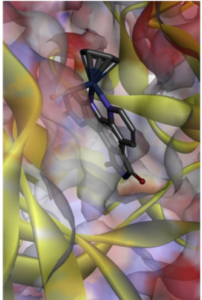
Figure 2: Osmium staurosporin-complex binding in the ATP binding pocket of Pim-1 kinase
More resent applications of the metal in medicine include photodynamic therapy (PDT), a less invasive strategy that leverages the photophysical and photostability properties of these osmium complexes and cell imaging using organelle-targeting luminescent probes.7 One major advantage of osmium over ruthenium is the relative stability of the coordination sphere with respect to ligand exchange or hydrolysis and subsequent inactivation of the complex. An important factor for any perceived in vivoapplications.
Several ruthenium-based molecules have been investigated clinically including IT-139 from Interzyne, currently in phase ½ for pancreatic and gastric cancers (Figure 3).8 However to date the are no osmium complexes in clinical trials.
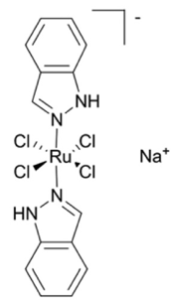
Figure 3: IT-139 (Interzyne), A ruthenium based anti-cancer complex currently in the clinic
Not surprisingly the medicinal application of osmium beyond the oncology field has had little traction. That said, if you look back far enough someone, somewhere will have probably given it a try. In 1980 the journal Rheumatologypublished results of a five-year study on the intra-articular application of osmic acid (synonym for osmium tetroxide) in rheumatoid arthritis patients.9 With few available disease-modifying treatments back in the 1970’s and the historical precedent of gold as an inflammatory cytokine modulator (since the 1900’s) it was probably not a bad thing to look at- under very close medical supervision of course.10 The results were somewhat disappointing, with good scores reported after 1 year declining significantly over the 5 year period. With the high degree of toxicity associated with osmium tetroxide the risks very much outweighed the benefits.
Osmium tetroxide is perhaps the compound most people associate with osmium. It’s a solid and surprisingly, given the density of osmium, is volatile. Exquisitely toxic, chronic exposure to low levels (below olfactory detection) can cause fatal pulmonary edema. The volatile oxide can also stain human corneas resulting in permanent blindness.11 A somewhat horrifying account of do-it-yourself toxicology can be found in paper published by McLaughlin et al in the British journal of industrial medicine.12 The paper describes experiments by F.R. Brunot in 1933, in which he deliberately exposes himself to OsO4vapour and documents the ill effects. Most notable were a “metallic taste in the mouth’ at the 10-minute timepoint and ‘smoking was unpleasant’. After 30 minutes he reports “a smarting sensation in my eyes” and at 3 hours a “a definite constriction in the chest and breathing was difficult”. He continued “Upon going out into the street the lamps appeared to be surrounded by large haloes as if there were a dense fog”. I’m sure the grim reaper was sharpening his scythe at his point. I don’t know what happened to Brunot- surely a candidate for a Darwin award….
If nothing else his fingertips should have become darker- osmium tetroxide is used as a lipid staining agent in microscopy and has been used historically to detect fingerprints. Presumably the osmium seeks out the unsaturation in the lipids and residual oils and does its thing.
Continuing in this insidious vein, in April 2004 British intelligence services foiled a plot to detonate an explosive device containing osmium tetroxide- a so called “dirty bomb”.13 This is bizarre on many levels, not least because the material would probably disintegrate during the blast and the osmium disperse so widely as to cause no acute risk to health. In addition, the high cost of the material (current price of osmium is $400/troy ounce- roughly 30g) and the rarity of the element would leave a trail back to the hapless perpetrator.
Osmium tetroxide is not all bad by any means. In organic synthesis it has a rich history and is utilized in arguably one of the most important catalytic asymmetric processes ever discovered- the asymmetric dihydroxylation reaction. In 1908 it was reported by Makowka that olefins could be cis-dihydroxylated using stochiometric quantities of OsO4. In 1912 Hoffmann described a major advance in this methodology- the use of metal chlorates as co-oxidants- enabling a reduction in the amount of toxic, rare and expensive osmium needed for the reaction. In 1936 Rudolf Criegee observed that addition of amines to the reaction mixture accelerated the rate and proposed a mechanism. In 1976 chemists at Upjohn Pharmaceuticals reported a procedure using catalytic osmium tetroxide and N-methylmorpholine-N-oxide as co-oxidant to generate racemic diols.14 Around the same time K. Barry Sharpless described the use of tert-butyl hydroperoxide as a co-oxidant, however in light of the Upjohn procedure this was never widely adopted. In 1980 Sharpless, building on the work of Criegee, reported the first asymmetric dihydroxylation using catalytic osmium and stochiometric chiral tertiary amines derived from the chinchona alkaloids.15 The final piece of the puzzle- the use of sub-stochiometric amounts of amine- was achieved with the introduction of pseudo-enantiomeric (DHQ)2PHAL and (DHQD)2PHAL diamine ligands (Figure 4). The diamine ligands significantly accelerated the rate of the process enabling use of catalytic quantities- so called ligand accelerated catalysis.
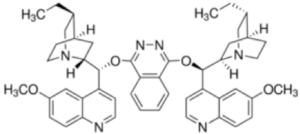
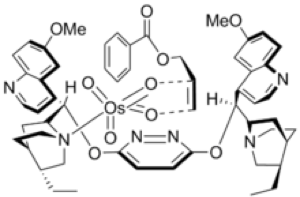
Figure 4: (DHQ)2PHAL ligand and its complex with Osmium
Refinements to the process continued over the next decade, with the introduction of potassium ferricyanide as co-oxidant and the use of the non-volatile potassium osmate salt (K2OsO4(H2O)2in place of OsO4. Addition of methanesulfonamide was also found to accelerate rate-limiting hydrolysis of the intermediate osmate (VI) ester when using the ferricyanide co-oxidant. A commercially available mixture of pre-mixed solids containing whichever ligand is predicted to give your required stereochemistry- the so-called AD-mix- was the final icing on the cake. I used AD-mix during my PhD for dihydroxylation of a protected allylic amine and it worked very nicely- thank you very much.
Major (heated) disagreements over the mechanism of the reaction raged for many years and makes interesting reading.15 In 2001 Sharpless shared the chemistry Nobel prize with Knowles and Noyori for his work on osmium catalysed reactions and ligand accelerated catalysis.16 Sharpless went on to develop the amino-hydroxylation reaction, first disclosed in 1996, with osmium again taking centre stage in the catalytic cycle.17
Further innovations to attenuate the possible toxicity exacerbated by volatility of the oxide include a polymer supported reagent prepared by Herrmann et al based on cross-linked poly(4-vinylpyridine)18a and immobilization of the oxide in a microreactor using poly(4-vinylpyridine) nanobrushes.18b Both appear to have come and gone without making a significant impact in the field. A quick check on the Sigma website revealed the former, once offered in their catalogue, has now been discontinued.
Our final delve into the chemistry of Osmium involves its use as a catalyst for asymmetric transfer hydrogenation. Sound a little boring? Perhaps. But what if I told you the reaction takes place in a cancer cell and could provide a new strategy for fighting the disease?
As one might expect the direct osmium analogue of the well-established and highly efficient Noyori ruthenium catalyst [Ru(p-cymene)(TsDPEN)Cl], (H)TsDPEN=N-p-tosyl-1,2-diphenylethylenediamine, gives very similar yields and ee’s in the asymmetric hydrogenation of aryl ketones.19 X-ray structures of the 16-electron Os (II) catalyst are almost identical to the Ru (II) species. The advantage of osmium systems are the apparent ease of synthesis and the additional hydrolytic stability of the complexes, particularly in cellular environments- a factor I mentioned earlier in the article (Figure 5).
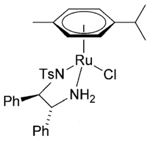
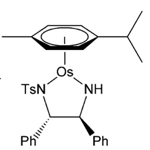
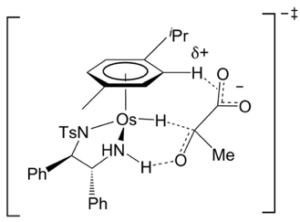
Figure 5: Noyori’s [Ru(p-cymene) (TsDPEN) Cl] catalyst, the Osmium analogue and the proposed transition state for the asymmetric hydrogenation of pyruvic acid to lactate
Will’s and Sadler at Warwick University have extended the application of transfer hydrogenation of prochiral ketones to reduction of intracellular substrates- namely the conversion of pyruvate to D-lactate- in both model aqueous systems and cancer cells.20 This utilizes the increased robustness of the osmium complex and uses sodium formate as a hydride source (Figure 5). The idea of targeting cancer cells is not the production of D- or L– lactate per se (these are non-cytotoxic natural products), but to perturb local concentrations of lactate in the cancer cell, or reduce levels of pyruvate, and in doing so inhibit or activate a key downstream signalling pathway that might prove fatal to the cancer cell. The team have shown that asymmetric reduction is possible (generating either enantiomer of lactate depending on the catalyst configuration) and to some extent demonstrated the biocompatibility of the catalyst. A significant hurdle, in common with many approaches of this type, is delivery of the catalyst (or pre-catalyst) and reductant to the cancer cell in vivo. The paper concludes with a few thoughts on this- nanoparticle encapsulation or polymeric micelles are suggested. Both are desperately difficult to achieve. This is an interesting idea and an innovative use of a metal that most people will never come across. Hiding in plain sight- it’s there on the table if you look hard enough.
Hope you enjoyed the article- See you next time.
References:
- a) John Emsley Nature’s Building Blocks: An A-Z Guide to the Elements, OUP Oxford, 2011 (p603-608); b) https://www.britannica.com/science/rhenium-osmium-dating.
- Is osmium always the densest metal? A comparison of the densities of osmium and iridium: Johnson Matthey Technol. Rev. 2014, 58(3), 137-141.
- Identification of an iridium-containing compound with a formal oxidation state of IX: Nature 2014, 514, 475-477.
- Structure-activity relationships between ruthenium and osmium anticancer agents- towards clinical development: Chem. Soc. Rev. 2018, 47, 909-928; Development of anticancer agents: wizardry with osmium Drug Disc. Today 2014, 19(10), 1640-1648; J. Coord. Chem. 2018, 71(2), 342-354; Mini Rev. med. Chem. 2016, 16(17), 1359-1373; The contrasting chemical reactivity of potent isoelectronic iminopyridine and azopyridine osmium (II) arene anticancer complexes: Chem. Sci. 2012, 3, 2485-2494; Osmium (IV) complexes as a new class of potential anti-cancer agents: Chem. Comm. 2011, 47, 2140-2142; Metallomics 2014, 6, 1014-1022.
- Future potential of osmium complexes as anticancer drug candidates, photosensitizers and organelle-targeted probes: Dalton Trans. 2018, 47, 14841-14854; ibid 2018, 47, 9934-9974.
- a) Intracellular localization of an osmocenyl-tamoxifen derivative in breast cancer cells revealed by synchrotron radiation X-ray fluorescence nanoimaging: Angew. Chem. Int. Ed. 2019, 58, 3461-3465; b) Organometallic antitumor compounds: ferrocifens as precursors to quinone methides: Angew. Chem. Int. Ed. 2015, 54, 10230-10233; c) For synthesis of the complex see Eur. J. Inorg. Chem. 2015, 25, 4217-4226.
- Photolabile ruthenium (II)–purine complexes: phototoxicity, DNA binding, and light‐triggered drug release: Eur. J. Inorg. Chem. 2017, 12, 1745-1752.
- Safety and activity of IT-139, a ruthenium-based compound, in patients with advanced solid tumours: a first-in-human, open-label, dose-escalation phase I study with expansion cohort: EMSOopen 2016, doi:1136/esmoopen-2016-000154.
- Rheumatology 1980, 19(1), 25-29.
- Gold was also used historically to treat TB, J. Public. Health 1925, 15(7), 631.
- Toxic tips: osmium tetroxide Chem. Health. Safety 2007, 14(5), 40-41.
- Toxic manifestations of osmium tetroxide: J. Ind. Med. 1946, 3(3), 183-186 (doi: 10.1136/oem.3.3.183)- free to download.
- http://news.bbc.co.uk/1/hi/uk/3603961.stm; https://www.newscientist.com/article/dn4863-experts-divided-over-poison-bomb-claim.html
- Discovery: Tett. Lett. 1976, 17(23), 1973-1976; scale-up examples: Sharpless asymmetric dihydroxylation on an industrial scale: Org. Process. Res. Dev. 1997, 1(6), 425-427;Org. Process. Res. Dev. 2003, 7(6), 821-827.
- Chem. Rev. 1994, 94(8), 2483-2547; Chem. Rev. 2002, 80(2), 187-213; Tett. Asym. 2017, 28(8), 987-1043; Angew. Chem. Int. Ed. 2002, 41, 2024-2032; J. Org. Chem.2009, 74(8), 3038-3047.
- Platinum Metals Rev. 2002, 46(2), 82-83.
- J. Chem. Soc., Perkin Trans. 1 2002, 2733-2746.
- a) Mol. Cat. A 1997, 120, 197-205; b) Angew. Chem. Int. Ed.2013, 52(26), 6735-6738.
- Easy to synthesize, robust organo-osmium asymmetric transfer hydrogenation catalysts: Chem. Eur. J. 2015, 21, 8043-8046.
- Asymmetric transfer hydrogenation by synthetic catalysts in cancer cells: Nature Chem. 2018, 10, 347-354.
Osmium by the numbers:
| Atomic number | 76 |
| Group | 8 |
| Atomic weight | 190.23 |
| No of Nat Isotopes (stable isotopes) | 7 (6) |
| Melting point | 3054°C |
| Boiling point | 5027°C |
| Density | 22.587 g/cm3 |








