Iridium is ubiquitous in organic synthesis. In the last decade alone we have seen its impact as a phtoredox catalyst for a wide range of light-driven reactions that are difficult to achieve using traditional synthetic methods.1 In addition, iridium has rich utility in C-H activation chemistry- a recent review by Cramer highlighting chiral complexes as catalysts for enantioselective functionalization of C-H bonds in wide range of molecular scaffolds.2
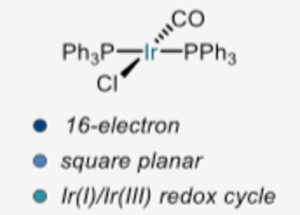
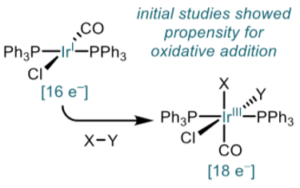
Vaska’s complex (Figure 1), the discovery of which is credited to Angoletta in 1958 but named after the Estonian-American chemist Lauri Vaska after his extensive characterisation in 1962, is an air-stable 16-electron square planar iridium (I) complex that readily undergoes oxidative addition with a number of oxidants, acids and electrophiles (e.g. CO, SO2,Cl2 , CH3I) generating an 18-electron octahedral Ir(III) species (Figure 2).Vaska’s complex also binds reversibly to molecular oxygen in a ‘side-on’ fashion as opposed to the ‘end-on’ binding found with the iron complex haemoglobin.3
Synthesis is relatively straightforward- heating Ir(Cl)3 hydrate with triphenylphosphine in DMF in an atmosphere of carbon monoxide. Alternatively, decomposition of DMF during the reaction is an indirect source of CO (heating above 160°C). In light of a recent DMF safety review Corteva however this is an ill-advised strategy.4 2-methoxyethanol has also used as a reaction solvent and source of CO. Fortunately (for you) the complex is commercially available.
So what can you do with it? Arguably the most attractive synthetic application is conversion of tertiary amides to highly substituted amines, particularly since partial C=O reduction generates a reactive intermediates (hemi-aminals, iminium ions and enamines) that can be trapped to generate a new C-C bond (Scheme 1).5 Traditional hydride reducing agents such as aluminium and boron species tend to generate tertiary amines, preventing the utilisation of partially reduced intermediates. Amide bond formations are by far the most frequently encountered reactions in medicinal chemistry, providing a rich vein of substrates for elaboration.6
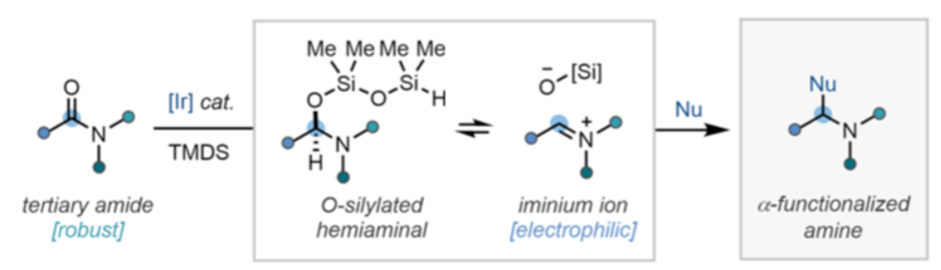
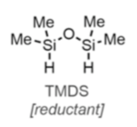
Hydrosilylation using Vaska’s complex in combination with tertramethyldisiloxane (TMDS), or its polymeric counterpart PMHS, has been used for chemoselective reduction of tertiary amides and synthesis of alpha-substituted amines, however mild partial reduction of amides (including N-methoxyamides) to O-silylated hemiaminals and downstream activated C=N intermediates- that can be trapped by nucleophiles- has attracted a number of research groups, including those interested in natural product synthesis and late stage diversification. The recent review by the Dixon group provides an excellent summary of what’s been achieved here.5
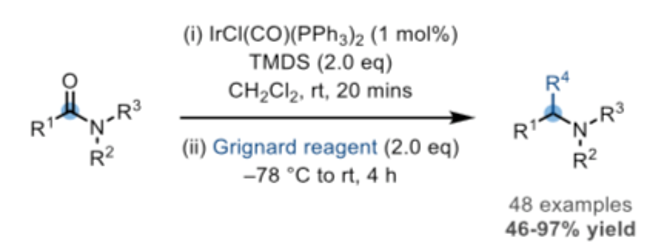
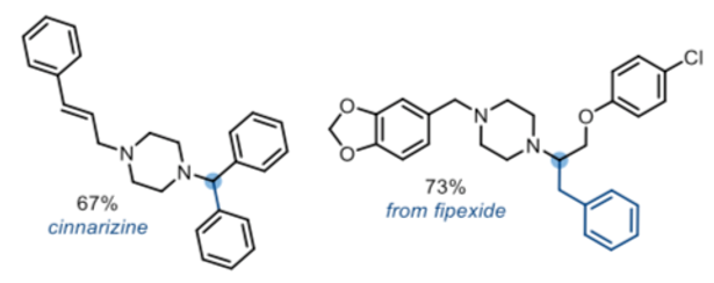
A number of reactions caught my eye from a process chemistry perspective. The first is a one-pot coupling of tertiary amides (and lactams) with Grignard reagents reported by the Dixon group in 2017, in which alpha-amino arylation, alkene/alykynylation and simple alkylation occurs in high regioselectivity (Scheme 2).7 Structural modification of a number of known API’s was used to outline the scope of the approach including reduction/benzylation of the amide carbonyl in the nootropic agent Fipexide and synthesis of the antihistamine Cinnarizine (Figure 3). Robustness of the approach was affirmed by publication of an Org. Synth. Procedure in 2019, run on 15 g scale.8
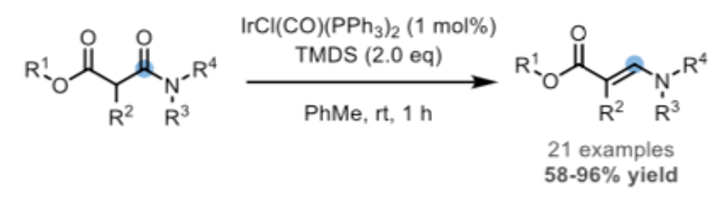
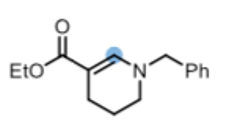
The second is the chemoselective reduction of β-amido esters to the corresponding β-enamino esters- widely used synthetic building blocks- in 2019 by Huang and Ye (Scheme 3).9 Cyclic β- amido esters were also reduced in this system to give endocyclic β-enamino esters such as the useful piperidine building block shown in Figure 4.
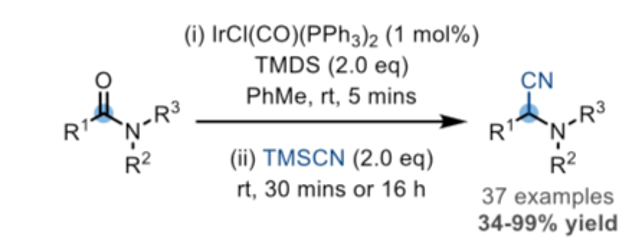
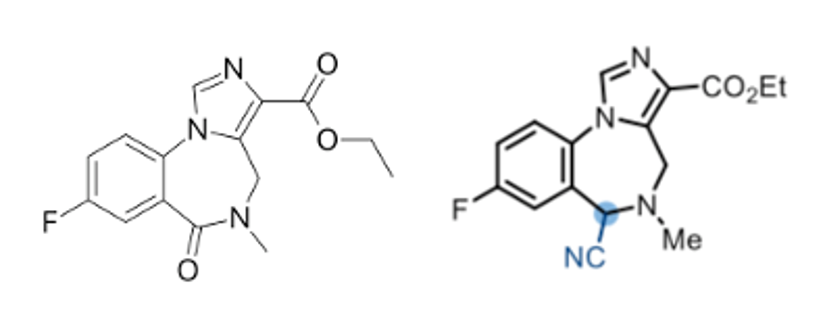
Finally Dixon and co-workers have developed a reductive Strecker reaction (Scheme 4) using Vaska’s complex in combination with TMDS and a cyanide source (TMS-cyanide).10 The alpha-aminonitrile products are precursors to the corresponding racemic amino-acids, though kinetic resolution using a nitrilase enzyme might expand the scope of this methodology.11 Late stage modification of the GABA-receptor antagonist Flumazenil was used to demonstrate the applicability of the methodology in modification of complex drug targets (Figure 5).
The combination of the air stability of Vaska’s complex and stability of TMDS (relative to many other hydride reagents) lends itself well to industrial application. The scope of TMDS as a reducing agent has been reviewed by Larson.12
References:
- Visible light photocatalysis: applications and new disconnections in the synthesis of pharmaceutical agents: C. Stephenson et al, Org. Process Res. Dev. 2016, 20, 1134-1147.
- Catalytic enatioselective functionalizations of C-H bonds by chiral iridium complexes: Cramer et al, Chem. Rev. 2020, 120, 10516-10543.
- Oxygen-carrying properties of a simple synthetic system: L. Vaska Science 1963, 140, 809-810.
- Potential safety hazards associated with using N,N-dimethylformamide in chemical reactions: Q. Yang et al, Process Res. Dev.2020, 24, 1586–1601.
- Catalytic reductive functionalization of tertiary amides using Vaska’s complex: synthesis of complex tertiary amine building blocks and natural products: D. Dixon et al, ACS catal. 2020, 10, 8880-8897.
- Analysis of past and present synthetic methodologies on medicinal chemistry: where have all the new reactions gone?: D. Brown et al, Med. Chem. 2016, 59, 4443−4458.
- Tertiary amine synthesis via reductive coupling of amides with Grignard reagents: D. Dixon et al, Chem. Sci. 2017, 8, 7492.
- Iridium-catalysed reductive coupling of Grignard reagents and tertiary amides: D. Dixon et al, Org. Synth.2019, 96, 511-527.
- Ir-catalyzed chemoselective reduction of b-amido easter: a versatile approach to b-enamino esters: P. Huang et al, Tetrahedron 2019, 75, 1624−1631.
- Iridium-catalyzed reductive Strecker reaction for late stage amide and lactam cyanation: D. Dixon et al, Angew. Chem., Int. Ed. 2017, 56, 3655-3659.
- Nitrilase: a promising biocatalyst in industrial applications for green chemistry: J. Shen et al, Rev. Biotech. 2021, 41, 72-93.
- Tetramethyldisiloxane: A practical organosilane reducing agent: G. Larson et al, Org. Process Res. Dev.2016, 20, 1164–1181.








