Five-membered heterocyclic arenes account for a large portion of the heterocycles found in pharmaceuticals and bioactive molecules.1 Synthesis of heteroaryl halides and elaboration via metal catalysed cross coupling reactions- particularly with aliphatic amines- is, however, known to be challenging. These small heterocycles-particularly electron-rich systems- frequently coordinate to palladium, promoting catalyst deactivation via displacement of the supporting phosphine ligand.2a-c Many are also unstable in the presence of the strong bases often encountered during synthetic chemistry and their decomposition produces small anionic fragments that can act as potent catalyst poisons.2a-c
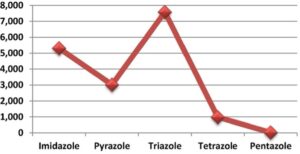
One very common aryl heterocycle used a fair amount in drug discovery are the triazoles. A search of the Scopus database by Aisa et al using the terms “imidazole”, “pyrazole”, “triazole” and “tetrazole” over a 10-year period from 2008-2018 revealed that triazoles, particularly 1,2,3-triazoles, were the most frequently studied azole ring system.3 This is largely due to advances in click chemistry and the facile assembly of the triazole ring by copper-catalysed azide-alkyne cycloaddition (Figure 1).4
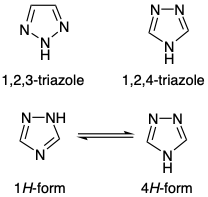
The isomeric forms of these useful intermediates often compound selective functionalisation of one or other of the reactive nitrogen atoms in the ring (Figure 2). From a scale-up perspective, triazoles can demonstrate significant thermal activity and, depending on their substitution pattern or method of assembly, require extensive process safety testing.5
Yet despite their importance and unique steric and electronic properties, methods of preparing these small, low molecular weight, heteroaromatic building blocks are notoriously challenging to carry out and, as eluded to above, often leads to mixtures that need extensive purification.2c Often they are water soluble placing an additional burden on the reaction work-up.
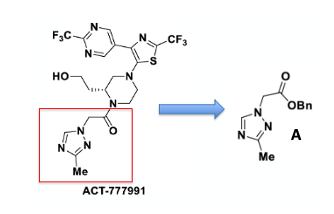
This article concerns the synthesis of a 1,2,4-triazole building block (A) required for the scale-up of the CXCR3 agonist ACT-777991, discovered and developed by Idorsia (Figure 3).6 The compound is currently in phase I clinical trials in combination with an anti-CD3 antibody for the treatment of type-1 diabetes.7
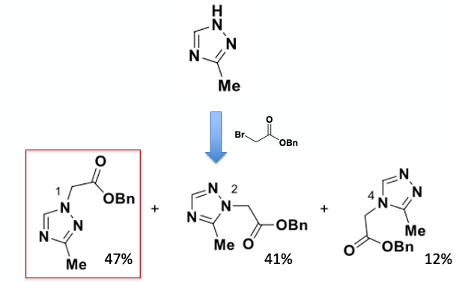
The first-generation route to the 1,2,4-triazole building block (A) was direct alkylation of commercially available 3-methyl-1H-1,2,4-triazole with benzyl 2-bromoacetate (K2CO3 / acetone). It’s an obvious place to start and would have been perfect if it was a selective process. However in reality it went just about as bad as it could have done- generating a mixture of predominantly the N1 and N2 regioisomers, with a lesser amount of the N4 alkylated material (Figure 4, ratio: 47:41:12). Separation of the required N1 isomer by column chromatography and subsequent recrystallisation gave a poor recovery of the required N1 isomer (17% on 1.6Kg scale). Hydrogenation of the benzyl ester gave the carboxylic acid in good yield (H2/Pd/C, 95%). This approach is fairly typical of an enabled discovery chemistry route- going with what you have to make enough material to keep the project moving forward. In its defence the step-count is low, however, the poor regioselectivity (non-existent if your being pragmatic), separation and purification were not sustainable for further development. A ‘green chemistry” continuous one-pot flow method was reported by Idorsia in 2020.8 I’m not aware of this being used at scale.
Time for a re-think. The two options available at this point were looking for an alternative, readily available, inexpensive starting material with the triazole ring already intact, or a more radical de-novo approach- constructing the ring system from first principles as it were (Figure 5).
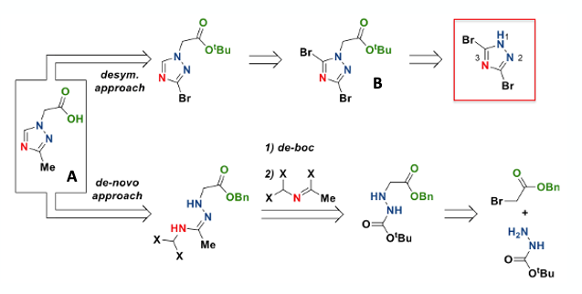
Starting from a commercially available stating material offers many advantages, probably the most important being that construction of the triazole ring using likely high energy intermediates is avoided.9 3,5-Dibromo-1H-1,2,4-triazole fitted the bill nicely (Figure 5). Alkylation of this Pseudo-symmetrical dihalide with tert-butyl-2-bromoacetate gave selective N1/N2 alkylation in 95% assay yield on a 700g scale (Figure 5 (B), K2CO3, AcCN, 40°C). The advantage here is that the N4-nitrogen atom is not alkylated. Intermediate (B) was telescoped directly into the next step, avoiding a formal work-up and isolation.
Now the key step- selectively burning out the C-5 bromine atom.10 There was literature precedent for this. A 2006 synthesis paper by Miethchen et al described selective reduction of a nucleoside bearing an N1– substituted dibromotriazole with dimethylphosphite (Figure 6).11 This is remarkable in that there is an additional bromine substituent on the D-mannopyranosyl fragment that remains untouched. There is a great deal of similarity here with a key reduction step in the manufacturing process toward Omecamtiv Mecarbil described in one of my earlier blog posts.12
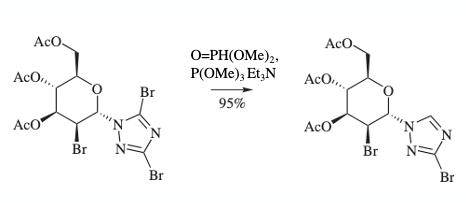
Repeating this method with our triazole (B) worked splendidly. The required mono-bromotriazole was obtained in 78% yield on 975g scale (1.1 eqv. P=OH(OMe)2, 2.5 eqv. NEt3, 5 vol MeCN, 70°C).
The penultimate step in the sequence was introduction of the methyl group (Figure 7). Plenty of options here, and the team hit the reaction very hard, carrying out extensive screening and optimisation. Most approaches involved Palladium-catalysed cross-coupling with various boron-based methylation reagents including trimethylboroxine, methylboronic acid, Me-MIDA boronate and the Molander MeBF3K reagent (which is apparently good for difficult alkyl transfers).13 Methyl nucleophile such as Me2Zn, MeLi and MeMgCl were also explored, the latter using a Fe(acac)3 catalyst. Turbo-Grignard magnesium exchange and alkylation with MeI was also investigated. None of these options worked particularly well, often leading to proteolytic debromination or formation of unidentified impurities.14
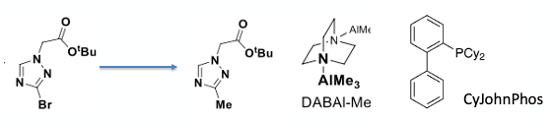
The team chose to optimise one of the better leads from the screen- DABAL-Me3 (1,4-Diazabicyclo[2.2.2]octan-bis-(trimethylaluminium) with Pd(dbd)3 and XPhos. A ligand screen and optimisation of reaction conditions identified CyJohnPhos in THF solvent as the best combination (Figure 7, 0.006 eqv. Pd(dba)3, 0.6 eqv. DABAL-Me3, 0.012 eqv. CyJohnPhos, THF (8 vol), 75°C), giving the methylated product in 83% yield on 211g scale. The reaction mixture is described as being dark purple in colour. A sight to behold I’m sure. DSC and PhiTec calorimetry showed the reaction had no safety concerns (other than methane production during the quench). DABAL-Me3 is a bench-stable solid that is nowhere near as horrific at AlMe3. As an aside, trimethylaluminium is the world’s largest tonnage organometallic- at least 10,000 tons/year. Who knew.15
Before I move on let’s look in more detail at the methylating agent. DABAL-Me3 must have skeletons in its closet. So once again I went digging. A paper by Woodward (no- not that Woodward) describes its preparation and properties.16 Apparently its easily handled under normal laboratory conditions and has hydrolytic stability comparable with LiBH4. It can be weighed in air and is a free-flowing white powder. It’s prepared by adding AlMe3 to DABCO and swirling the resulting solid in diethyl ether. There is a warning in the paper, however. A combination of residual AlMe3 and diethyl ether are not good bed fellows and can ignite. An interesting comment in the paper is that [DABAL-Me3] “ignites on tissue paper, especially on damp days”. If the process R&D labs at Idorsia in Switzerland share the same weather fronts as we here in the UK they must get a lot of damp days. But hopefully no fires. The material is commercially available, but I have no idea at what scale it is manufactured. There is a YouTube video as part of the excellent “periodic videos” series (created by Nottingham university) that shows Woodward playing with pyrophoric AlMe3 and handling the much less feisty DABAL-Me3.17
As with most aluminium based reactions the work-up needed a great deal of attention. A couple of things that proved to be important were the stability of the tert-butyl ester during acidification of the reaction mixture, and the methane off-gassing. Using aq. HCl resulted in significant cleavage of the tert-butyl ester, however 2M aq. AcOH resulted in no hydrolysis. The off-gassing of a non-condensable gas (CH4) is a problem at scale. Attempts to quench the DABAL-Me3 using a scavenging-type approach by methylation of added reactive electrophiles (aldehydes, nitriles etc.), though not a bad idea in principle, proved ineffective. The definitive work-up came from additional development of the acetic acid quench. The final process involved dilution with iPAc and carrying out an inverse quench onto 2M AcOH. The aluminium salts remained in the aqueous phase. A solvent switch to toluene and continuous distillation chased out the residual AcOH, then an anti-solvent induced crystallisation of the product was carried out by adding heptane. The inverse quench gave better control of the methane release. A strong nitrogen flow prevented formation of flammable mixtures.
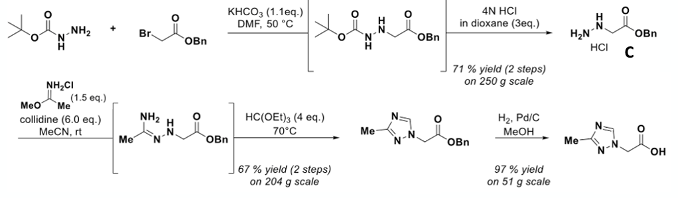
The final approach to acid intermediate (A) is a de-novo approach shown in Figure 8. It’s a 3-step approach involving the generation and isolation of hydrazine intermediate (C). This material demonstrated a strong exothermic decomposition in the DSC (-794 J/g) with a relatively low onset temperature of 135°C. The material was, however, shown to be shock insensitive.6 The compound could not be telescoped into the next synthetic (condensation) step so solvent-wet solid was isolated and processed. The remaining two steps were uneventful.
A cost analysis of the various synthetic routes showed a clear advantage in the de-novo assembly of the triazole. The desymmetrization approach was estimated to provide intermediate at twice the cost per Kg ($8,000/Kg vs $4,000/Kg) over the same number of isolation steps due to the use of Pd2(dba)3 and the unusual methylating agent, even though the overall yield of the former was higher (59% vs 46%). As a frame of reference, the 1st generation route had an overall yield of 16% over 2 isolation steps (and a difficult separation) with an estimated cost of $15,000/Kg.
I think from a safety perspective it’s unlikely that isolation of intermediate (C) is a sustainable approach to the manufacture of the triazole. A continuous flow process might be the way forward or finding a way to enable a fully telescoped process.
A nice storey demonstrating how synthesis of a seemingly simple intermediate can become a significant undertaking leading us into truly tri(azol)ing times.
See you next time.
References:
- a) Rings in drugs: R. Taylor et al, Med. Chem.2014, 57, 5845–5859; b) 1,2,4-triazoles as important antibacterial agents: P. Swiatek et al, pharmaceuticals 2021, 14, 2
- a) A bulky biaryl phosphine ligand allows for palladium-catalyzed amidation of five-membered heterocycles as electrophiles: S. Buchwald et al, Chem. Int. Ed. 2012, 51, 4710-4713; b) Pd-catalyzed amination of base-sensitive five-membered heteroaryl halides with aliphatic amines: S. Buchwald et al J. Am. Chem. Soc. 2023, 145, 3323–3329; c) Data-rich experimentation enables palladium-catalyzed couplings of piperidines and five-membered (hetero)aromatic electrophiles: M. Sather et al, Org. Process Res. Dev. 2019, 23, 1725-1739
- 1,2,3-Triazole-containing hybrids as leads in medicinal chemistry: A recent overview: H. Aisa et al, Bioorg. Med. Chem. 2019, 27, 3511-3531
- Applications of click chemistry in drug discovery and development: B. Gopalan et al, 2016,https://doi.org/10.1002/9783527694174.ch2
- Thermochemistry and Initial decomposition pathways of triazole energetic materials: P. Zhou et al, Phys. Chem. A, 2020, 124, 2951–2960
- Development of a scalable route toward an alkylated 1,2,4-triazol, a key starting material for CXCR3 antagonist ACT-777991: R. Davenport et al, Org. Process Res. Dev. 2023, 27, 928-937
- a) Discovery of clinical candidate ACT-777991, a potent CXCR3 antagonist for antigen-driven and inflammatory pathologies: E. caroff et al, Med. Chem.2023, 66, 4179–4196; b) Crystalline form of a piperazinyl-thiazole derivative: WO2022162017; Hydroxyalkyl-piperazine derivatives as CXCR3 receptor modulators WO2016113344; 4-(benzoimidazol-2-yl)-thiazole compounds and related aza derivatives: WO2013114332; (R)-2-methyl-piperazine derivatives as CXCR3 receptor modulators: WO2016113346
- Development of an efficient and sustainable synthesis of 2-(3-methyl-1H-1,2,4-triazol-1-yl) acetic acid under continuous-flow conditions: G. Vile et al, Green Chem. 2020, 22, 3748-3758
- Synthesis methods of 1,2,3-/1,2,4-triazoles: a review: Z. Liu et al, Front. Chem. 2022, 3389/fchem.2022.891484
- Synthetic methods for the hydrodehalogenation of halogenated heterocycles: G. Chelucci et al, Curr. Org. Chem. 2012, 16, 2921-2945
- Synthesis and reactivity of halogenated 1,2,4-triazole nucleoside analogues with high potential for chemical modifications: R. Miethchen et al, Synthesis 2006, 3, 496-508
- Now you see me, now you don’t- a judicious work-around for over-bromination at benzylic carbon: J. Studley 2022, https://www.scientificupdate.com/process-chemistry-articles/now-you-see-me-now-you-dont-a-judicious-work-around-for-over-bromination-at-benzylic-carbon/
- Potassium trifluoroborate salts as convenient, stable reagents for difficult alkyl transfers: G. Molander et al, Opin. Drug Discov. Devel. 2009, 12, 811–823
- Recent advances in methylation: a guide for selecting methylation reagents: Y. Chen, Eur. J.2019, 25, 3405-3439
- Trimethylaluminum: K. Suzuki et al, e-EROS Encyclopaedia of Reagents for Organic Synthesis 2007, https://doi.org/10.1002/9780470842898.rt265.pub2
- Remarkably stable (Me3Al)2⋅dabco and stereoselective nickel-catalyzed AlR3(R=Me, Et) additions to aldehydes: S. Woodward et al, Angew. Chem. Int. Ed. 2005, 44, 2232-2234
- YouTube AlMe3: https://www.google.co.uk/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&ved=2ahUKEwj37KyI0deAAxUtV0EAHc1SC1oQtwJ6BAgTEAI&url=https%3A%2F%2Fwww.youtube.com%2Fwatch%3Fv%3DRfeaffoWajc&usg=AOvVaw0aOnz2ll4lGen_9Jn1mG-J&opi=89978449








