One of the most challenging reactions we carry out as synthetic chemists is bromination of a benzylic carbon- the so called Wohl-Ziegler reaction.1 It’s a seemingly simple transformation – not particularly difficult to perform- and potentially very useful. In fact it’s a little too easy. Just add NBS and a radical initiator to an aryl methyl (tolyl) substrate and the over-zealous bromide radicals jump into a feeding frenzy- giving a mixture of mono– and bis– halogenated products (amongst other things). The problem then is two-fold. How to control the process to get better selectivity (mono- v’s bis-) and how to purify the product mixture. Most of the time the mono-bromide is the required material- a useful (albeit potentially mutagenic) intermediate used generally for C-, N-or O-alkylation reactions or as a precursor to organometallic reagents used in cross-coupling chemistry.2 A modification to the Wohl-Ziegler reaction has been described using 1,3- dibromo-5,5-dimethylhydantoin (DBDMH) as a bromination reagent in the presence of catalytic ZrCl4. This combination prevents the competing bromination of the aromatic ring sometimes found with Brønsted acids.3
The generally accepted mechanism of the Wohl-Ziegler reaction, proposed by Goldfinger in 1953 (the chemist not the Bond villain) involves generation of bromine radicals from reaction of the radical initiator with bromine. Low concentrations of bromine are formed by an ionic reaction of NBS with the HBr by-product.4 An alternative mechanism proposed by Bloomfield in 1944, involving succinimidyl radicals generated from NBS, is not believed to be correct. Bloomfield’s hypothesis relied upon an assumption that the bond dissociation energy of the N−Br bond in NBS was significantly lower than that of Br−Br bond in molecular bromine. This was was later shown to be incorrect.
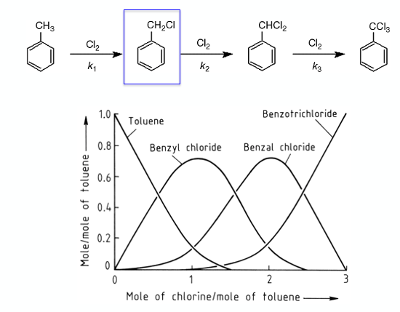
Graph 1 shows the course of the analogous radical chlorination of toluene (under batch photochlorination conditions using chlorine gas) and highlights the challenge of selective radical halogenation and more specifically the change in mixture composition as a function of chlorine equivalents. Under just about all reaction conditions it is impossible to change this product distribution- the required benzyl chloride intermediate containing both polychlorinated bis- and tri-chlorinated impurities (amongst other things).5 Only the reaction rate can be controlled by optimising the reaction conditions.
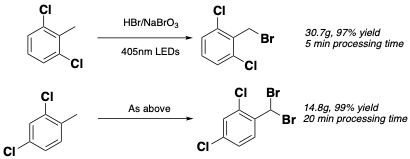
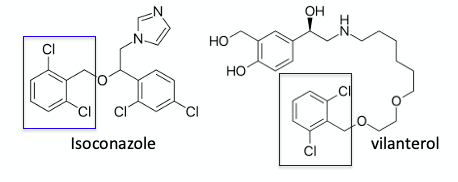
Continuous photochemical bromination, using in situ– generated bromine, has been described by Kappe et al.6 Bromine in this case is generated using NaBrO3/HBr and 405nm irradiation using LEDs initiates formation of bromide radicals. Specific dichloro-aryl substrates are exemplified in this paper: 2,6- and 2,4- dichlorotoluene. The dice is somewhat loaded in this case, with the di-ortho substrate since the rate of mono-bromination is much faster than formation of the dibromo compound (presumably due to steric reasons). Without the second ortho-chlorine atom, selective mono-bromination of the 2,4-isomer remains challenging, however, conversion to the dibromide- by increasing the residence time in the flow reactor and raising the temperature-gives good yields of the benzal devivative (Figure 1). There are a several APIs containing an ortho-dichloroaryl motif- the antifungal agent Isoconazole and the b2-adrenoreceptor agonist Vilsnterol are examples (Figure 2).
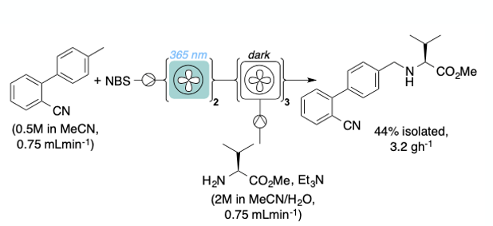
The Kappe group have also published a photochemical flow chemistry process using both BrCl3 as a bromine source.7 A recent paper by Marsden and Blacker et al describes a photochemical continuous stirred-tank reactor (CSTR) for continuous benzylic bromination, with the added advantage that the system can process solid materials and slurries.8 They demonstrate the utility of the system by running a telescoped photochemical bromination /nucleophilic substitution sequence to generate an intermediate for the antihypertension drug Valsartan (Figure 3).
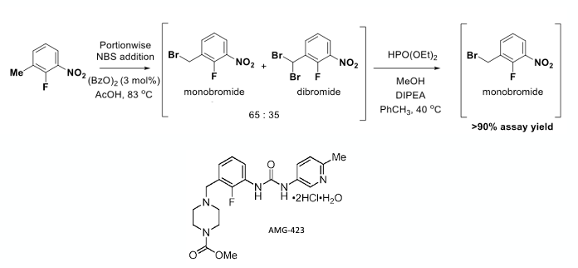
Where am I going with all this? Well an interesting series of papers published by Seb Caille and his team at Amgen describe development of a robust radical benzylic bromination process (using 2-fluoro-3-nitrotoluene as substrate) to generate an intermediate used in the commercial manufacture of their first in class myosin activator AMG 423 (Omecamtiv Mecarbil , Figure 4).9 The thing that is ingenious here is accepting that production of a mixture of the mono– and di– bromo products is inevitable, however taking that mixture and using a reductive process to selectively convert the over-brominated material to the required mono-bromo species puts less demand on developing conditions for selective mono– benzylic bromination and eliminates the need for tedious purification. Other industrial processes based on this bromination/reduction sequence have been used to functionalise inexpensive toluene feedstock materials.10
Treating a solution of the crude mono/di-brominated mixture in toluene with diethyl phosphite (0.46 eqv) in methanol in the presence of base (DIPEA) at 40°C for 3hrs gave full conversion to the required mono-bromide in >90% assay yield (Figure 4)!
There are a couple of older papers that set the precedent for this work. In particular, a paper by Deng et al from 2001 describes the debromination of polybrominated mixtures in some detail.11 Also a paper by Ohshiro et al in 1981 describes selective removal of bromide from gem-dibromoalkenes using diethyl phosphite, in this case generating mono-bromo alkenes.12a A slightly later paper describes in detail reduction of organic halides with diethyl phosphonate and triethylamine.12b
The first question to ask regarding a possible mechanism for this process is why does the reaction stop at the mono-bromo stage? In email correspondence with Seb Caille, his view is that the mechanism is anionic and that the selectivity is based on the stability of the anion formed by the reduction vs that which would be formed by reduction of the mono-bromide species.10
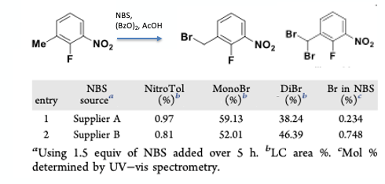
In a detailed kinetic and mechanistic study on the mono-bromination of 2-fluoro-3-nitrotoluene with acidic NBS (using benzoyl peroxide initiator) carried out by the Amgen team, continuous addition of a slurry NBS was demonstrated to minimize impurity formation and polymerization by maintaining a low solution phase concentration of Br2. An interesting finding was that different lots of NBS from two different suppliers gave different ratios of the mono– and bis– bromomethyl products (Figure 5). One of principles we strongly advocate in our process development and safety training courses is to establish the effect of impurities on a chemical reaction, particularly previously unknown impurities that might be introduced into an existing process stream via new batches of raw materials, reagents, or solvents. In this case NBS from Supplier B resulted in an increase of >8 LC area % of dibromide in comparison to the optimisation studies carried out using NBS from Supplier A. The most likely reason for this is that NBS lots with elevated levels of Br2/HBr impurity were more active in the radical bromination reaction due to the inherent rate- determining effect of these species in the reaction mechanism.
Word to the wise- a mixture of NBS, (BzO)2 and acetic acid is an extremely corrosive. Exposure of any metal in the reactor (including Hastelloy) results in significant damage. In the paper they show images of a Hastelloy impeller that will no longer be in active service.
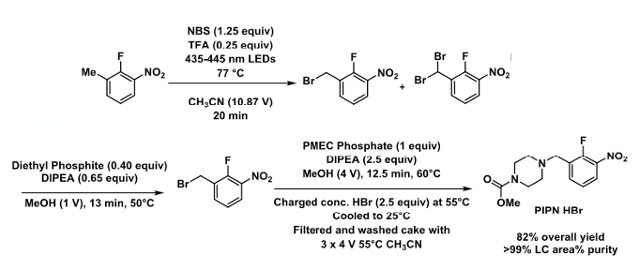
Leveraging these findings, the Amgen team subsequently developed a continuous photochemical bromination/reduction sequence (Figure 6). The advantages of a continuous process were that reaction times were shorter, the aqueous work-up was eliminated from the sequence (the flow stream was directly coupled with the downstream alkylation step) and the product crystallized directly from the crude reaction mixture.13 Lab studies showed a crude piperazine nitrotoluene hydrochloride salt could be prepared at rates of 25g/hr with a total end to end residence time of approximately 45 min. The use of continuous NMR analysis is highlighted as a powerful technique for kinetic analysis and optimisation of reactions of this type.
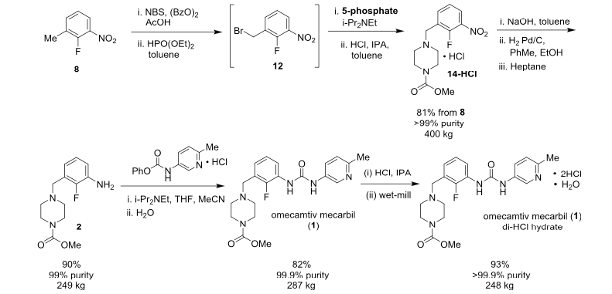
The pilot process used to prepare the API is shown in Figure 7. This route was ultimately used to prepare over 1 metric ton of drug substance.
I think you’ll agree this is an interesting approach to the manufacture of a key process intermediate- something I wish I’d been aware of when trying to selectively mono-brominate some tricky tolyl- derivatives in my past.
See you next time.
References:
- Brominations with N-bromosuccinimide and related compounds. The Wohl-Ziegler reaction: C. Djerassi, Rev.1948, 43, 271–317; Use of bromine and bromo-organic compounds in organic synthesis: P. Phukan et al, Chem. Rev. 2016, 116, 8312; Methods for bromination of benzylic positions: S. Zhao et al, Curr. Org. Chem. 2018, 22, 2444-2459.
- Utilization of methylarenes as versatile building blocks in organic synthesis: K. N. Singh et al, Chem. Soc. Rev., 2015, 44, 8062-8096.
- Lewis acid catalyzed benzylic bromination: K. Shibatomi et al, Chem. Asian. J. 2008, 3, 1581-1584.
- Laws of addition and substitution in atomic reactions of halogens: P. Goldfinger et al, Nature 1953, 171, 704-705; ibid 1951, 168, 30-32; N-Bromosuccinimide. Mechanisms of allylic bromination and related reactions, J. Incremona et al, J. Am. Chem. Soc.1970, 92, 627–634.
- Benzyl chloride and other side-chain-chlorinated aromatic hydrocarbons: K. A. Lipper et al, In Ullmann’s Encyclopedia of Industrial Chemistry, (Ed.). 2017, https://doi.org/10.1002/14356007.o04_o01.pub2
- Continuous photochemical benzylic bromination using in situ generated Br2: process intensification towards optimal PMI and throughput: O. Kappe et al, Green Chem., 2020, 22, 448 -454.
- Photochemical benzylic bromination in continuous flow using BrCCl3 and its application to telescoped p-methoxybenzyl protection: O. Kappe et al, Biomol. Chem., 2019, 17, 1384-1388; Continuous flow photochemical benzylic bromination of a key intermediate in the synthesis of a 2-oxazolidinone: O. Kappe et al, ChemPhotoChem 2018, 2, 906-912.
- Readily reconfigurable continuous-stirred tank photochemical reactor platform: S. Marsden & J. Blacker et al, Process Res. Dev. 2022, 26, 215–221.
- Kinetic investigations to enable development of a robust radical benzylic bromination for commercial manufacturing of AMG 423 dihydrochloride hydrate: J. Murray et al, Process Res. Dev. 2020, 24, 1523-1530; Development of a factory process for omecamtiv mecarbil, a novel cardiac myosin activator: S. Caille et al, Org. Process Res. Dev. 2019, 23, 1558-1567.
- Seb Caille, Amgen. Personal communication August 2022.
- An efficient method for the preparation of benzylic bromides: J. Deng et al, Synthesis 2001, 14, 2078-2080.
- a) Reduction of gem-dibromides with diethyl phosphite: Y. Ohshiro et al, J. Org. Chem. 1981, 46, 3745-3747; b) Reduction of organic halides with diethyl phosphonate and triethylamine: Y. Ohshiro et al, Chem. Soc. Jpn. 1983, 56, 1881-1882.
- Development of a Continuous Photochemical Bromination/Alkylation Sequence En Route to AMG 423: K. Quasdorf et al, Process Res. Dev. 2022, 26, 458-466.








