Organolithium reagents are powerful and ubiquitous intermediates used extensively in synthetic chemistry both in academic and industrial settings. The stability of these reagents often necessitates generation and rapid processing at cryogenic temperatures: some organolithium species are chemically or configurationally unstable at temperatures above -100°C. An example is the dichloromethyllithium anion generated during the Mattison homologation reaction used in the synthesis of Vaborbactam (Figure 1).1 The need for low temperature generation and processing of the organometallic species stems from its instability at higher temperatures, resulting in decomposition via formation of a carbene through a-elimination of LiCl.
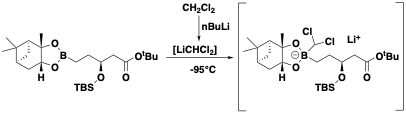
Scale-up of low temperature organometallic chemistry in batch is intrinsically challenging for a number of reasons. The ability to remove heat from the reactor during exothermic additions at cryogenic temperatures is difficult and often very high dilutions and slow addition rates are required to maintain control. This in turn can decrease efficiency and productivity. Poor mixing, particularly at the point of addition, can also generate localised hot-spots and variable local stoichiometries resulting in formation of impurities and impacting yield and product quality.
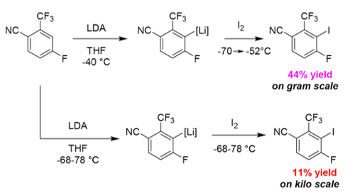
A good industrial example of these problems is publised in a recent(ish) paper by GSK describing the formation and subsequent iodination of a rapidly equilibrating aryl lithium species (Figure 2).2 On small scale in batch the yield was around 40%, which upon scale-up (to Kg scale) plummeted to 11%. Their (quite reasonable) hypothesis here was that the ArLi species was unstable under the reaction conditions-longer addition times and localised hot-spots in the reactor resulting in decomposition and formation of impurities (Figure 2).
Continuous processing is frequently used in scaling up chemistry that utilises highly reactive organometallic reagents such as organolithium or organomagnesium derivatives, including unstable species such as the lithium carbenoids referred to in the Matteson reaction described above. The Mattison process has been run continuously on production scale by a team at Patheon, Linz using a pipe reactor, with lithium anion generation actually possible at a higher temperature (-78°- -45°C) because of rapid (10’s seconds) down-stream processing.3 The benefits of a flow process (control of heat-flow, mixing and rapid reaction times) directly address the problems encountered when scaling in batch reactors. Yoshida et al have in the past advocated so called “flash chemistry,” which allows the generation and use of short-lived lithiated intermediates on the millisecond time scale, opening up reaction space almost impossible to achieve under conventional batch conditions.4
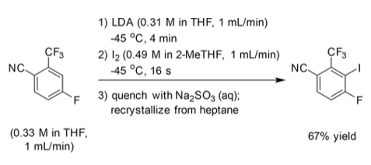
In the GSK iodination reaction descried above a continuous process was developed that was able to process material at a rate of 425g L-1h-1 in 67% yield. In this case the short reaction times and tight control over temperature and stoichiometry facilitated the generation and equilibration of an unstable lithium-intermediate (Figure 3). Equilibration of the kinetic product (5-lithio) to the thermodynamically more stable species (3-lithio) was fast and rapid quenching with iodine was important to maintain a high yield.
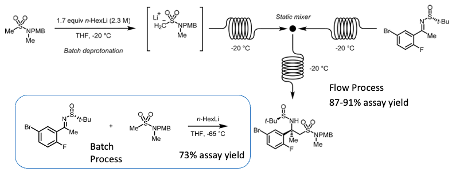
Continuous processing is frequently used to mitigate problems of this type encountered during batch processing. A good review on the use of organolithium bases in flow chemistry was published in Org. Process Res. Dev. by McGalcken et al in 2020.5 One of the case studies we use in our Chemical Development and Scale-Up course is the use of a flow lithiation reaction to produce the beta–-secretase inhibitor Verubecestat, a drug developed by Merck for the treatment of Alzheimer’s disease which unfortunately, along with several other companies’ efforts in this area, was unsuccessful. A flow process was developed to prepare a key process intermediate obtained by addition of a sulfone anion to a chiral imine (Figure 4). In the batch reaction poor assay yields were obtained. This improved significantly with a continuous process (from 73% assay yield to 87-91%, Figure 4). More than 100 kg of product produced at pilot plant scale using a pipe reactor.6
It’s not all sunshine, however. One of the issues frequently encountered in flow lithiation chemistry is fouling caused by the precipitation of lithium salts (LiOH) and the potential for fully blocking the system and over pressurising. This obviously has an impact on safety as well as productivity. Continuous processing relies on achieving a steady state equilibrium for robustness (a regulatory requirement for GMP manufacture)- something that can be difficult if solids are precipitating in the reactor over time. These salts can be removed at the end of a campaign by flushing with water, followed by a water miscible solvent (such as THF). This, however, introduces challenges relating to retained residual low levels of water (which can obviously result in the formation of more LiOH), the use of very large volumes of wash solvent and the physical properties of water- namely its low freezing point. Decomposition of organolithium reagents such as nBuLi on storage can also result in formation of insoluble lithium hydroxide . The solubility of LiOH (anhydrous) in water is 12.8g/100ml at 20°C.
Where’s this going? A recent paper by Machida and his team at Kaneca/Osaka descries a water-free process for cleaning the flow reactor by generating organolithium salts that are soluble in organic solvents.7
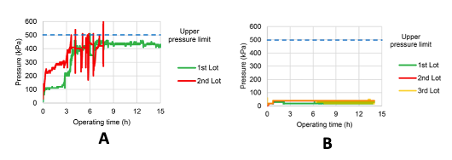
The blocking problem is nicely demonstrated in this paper with a case study on the continuous preparation of 100’s Kg of an acetal intermediate used in the manufacture of the diabetes drug Dapagliflozin (Figure 5, showing acetal lithium ion prior to acid quench).8 During the initial production campaign, processing the first lot of material resulted in a rapid system pressure increase after around 3 hours of continuous operation. This could be controlled by adjusting the feed rate below the upper pressure limit of the pumps (<500 kPa, Figure 6). Washing the reactor with water and THF followed by processing of the second lot of material resulted in a rapid initial pressure spike upon restarting the pumps, followed by several subsequent pressure spikes above the pump threshold limit (Figure 6, A). The issue was obviously the build-up and fouling of the reactor with precipitated lithium hydroxide and the solution (if you’ll pardon the pun) would be to remove the solid without introducing any water into the reactor.
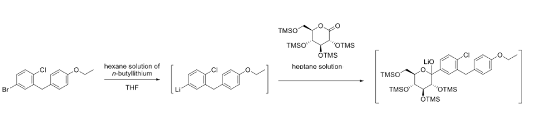
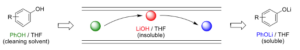
The idea they explored is a relatively simple one (most of the best ideas usually are). Pump a THF solution of a (weakly acidic) phenol or benzoic acid through the reactor, thereby converting the LiOH to THF-soluble lithium phenoxides (Figure 7). To this end the solubility of LiOH in a range of phenol/THF solutions were investigated (Figure 8).
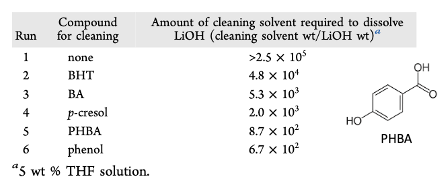
A THF solution of Phenol and PHBA (p-hydroxybenzoic acid) proved most effective based on the amount of cleaning solvent required to flush the system. Considering Pka values, PHBA was expected to be present as the lithium carboxylate. The increased solubility with respect to pure benzoic acid suggest the phenolic functional group was also having a solubilising effect.
Did it work? A second production run comprised of 3 lots of bromide was carried out, with the specification of the water in the aryl bromide feed solution set at 95ppm or below (after drying over sieves). No pressure increases were observed after implementation of the PHBA/THF cleaning protocol after the first and second lots (Figure 6, B).
I’m sure many companies have their own cleaning protocol for their own specific systems. I think this is a good publication ,of interest to the wider flow chemistry community. Always very satisfying to see potentially complex problems solved with simple answers.
See you next time.
References:
- Homologation of boronic esters to a-chloro boronic esters, D. Matteson et al, Organometallics, 1983, 2, 1529-1535; a-halo boronic esters: intermediates for stereodirected synthesis, D. Matteson Chem. Rev. 1989, 89, 1535-1551
- Selective continuous flow iodination guided by direct spectroscopic observation of equilibrating aryl lithium regioisomers: A. Dunn et al, Organometallics 2019, 38, 129-137
- Development of a continuous flow process for a Matteson reaction: from lab scale to full-scale production of a pharmaceutical intermediate: C. Schuster et al, Org. Process Res. Dev. 2019, 23, 1069–1077
- Flash chemistry: fast organic synthesis in microsystems: J. Yoshida, John Wiley & Sons, Ltd, 2008
- Organolithium bases in flow chemistry: A review: McGlacken et al, Org. Process Res. Dev. 2020, 24,1814–1838
- Development of an organometallic flow chemistry reaction at pilot- plant scale for the manufacture of verubecestat: D. Thaisrivongs et al, Org. Process Res. Dev. 2018, 22, 403−408
- Efficient cleaning method for flow reactors in flow lithiation reactions under water-free conditions: K. Mchida et al, Org. Process Res. Dev. 2023, https://doi.org/10.1021/acs.oprd.3c00321
- A concise and efficient synthesis of Dapagliflozin: J. Yu et al, Org, Process Res. Dev. 2019, 23, 1458− 1461








