The first electrophilc aromatic substitution reaction you were taught as an undergraduate was almost certainly nitration.1 Nitration and subsequent reduction of the aryl nitro group to an aniline via high temperature metal catalysed hydrogenation remains an important industrial process. The reduction has many flavours- both in terms of the substitution pattern on the aryl (and heteroaryl) ring and the choice of reducing agent, the latter stemming from the inevitable development of newer, milder methods. A good review on the subject was published in 2018 by Benaglia and Jost.2 The first industrial process for the reduction of nitrobenzene to aniline was developed by Antoine Béchamp in 1854, using iron as the reductant- the so called Béchamp reduction.3 The aniline products became raw material for the dye industry and subsequently the first soiree into medicinal chemistry by Paul Ehrlich.4
Anilines are precursors to elaborated aromatics via diazotisation- a process first described by Griess (a brewer most of his working life!) in 1858.5 An important milestone in this chemistry was reported by Sandmeyer in 1884, opening the door to a wide range of aryl functionalization reactions- most notably halogenation.6 These reactions are now run at considerable scale.7
Back to that reduction reaction, and a couple of recent papers that are of interest. The first is not a new approach but is noteworthy in that it builds upon existing methodology. The use of boron reagents in nitro group reduction has been reported several times over the last few years. I’m referring here to non-metal catalysed processes and the boron reagent acting as the putative reducing agent. These methods include bis(pinacolato)diboron in basic alcoholic solvents at 110 °C,8a and hypodiboric acid (B2(OH)4) in neutral water at 80 °C. 8b B2(OH)4 in combination with aqueous ethanolic NaOH is sufficiently mild for the reduction of a nitro groups in DNA−chemical conjugates.9
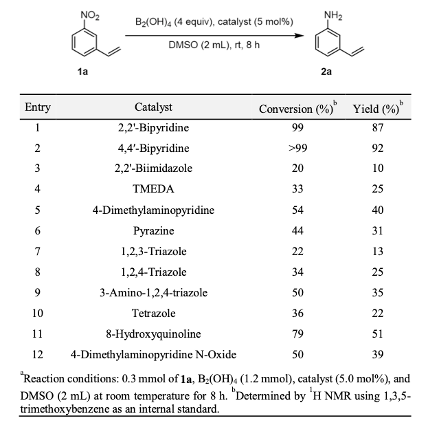
As a general method, bis– (pinacolato)diboron (B2pin2) and KOtBu at 110 °C works well, however the limitation of this methodology is that high temperatures and excessive amounts of base are required and only provide limited chemoselectivity. More recently Hosoya et al reported the reduction of nitro groups using B2(OH)4 or bis– (neopentylglycolato)diboron (B2nep2), catalysed by an organocatalyst, 4,4′- bipyridine.10 The latest addition to this area from Han et al is essentially combining methods and using as B2(OH)4 reductant with 4,4′-bipyridine as the organocatalyst.11 Formation of a biboron / Lewis acid ate-complex is most likely at work here. Screening inorganic and organic bases using 3-nitrostyrene as a bifunctional (nitro/alkene) substrate revealed that organic bases gave better selectivity v’s olefin reduction, and several of the organic bases showed catalytic activity at concentrations of 5mol% (Table 1). 4,4′-bipyridine provided the aniline reduction product in highest conversion (>99%) and in the highest yield (92%) when DMSO was used as the reaction solvent. Coincidence that the same bipyridyl catalyst as reported in the Mashima paper,10 is the best Lewis acid in this system? More to it than that I think. Switching solvent to DMF resulted in a very rapid increase in reaction rate, from 8hrs with DMSO to 10 minutes with DMF. Not sure what is driving this effect- and they don’t speculate in the paper.
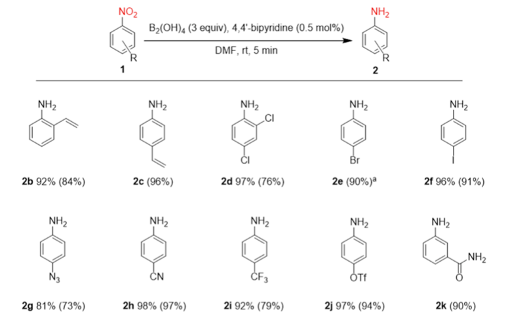
Challenging functional groups, such as vinyl (−C=CH2), internal alkene (C=C), ethynyl 2 carbonyl (C=O), nitrile, and halides (C−X) were all well tolerated, and the nitro reduced products obtained in high yields (Table 2). Heating the reaction mixture once the reduction was complete enabled the generation of the N-formyl derivative- easier to handle and isolate I guess. Not something I would recommend if running at scale- DMF decomposition at elevated temperatures has significant safety implications. A recent OPRD paper by Corteva gives plenty of details and recommendations.12
Deuterium labelling experiments (using d7 DMF revealed that the H atoms in −NH2 did not originate from DMF. Using B2(OD)4 revealed that the H atoms were derived from the boron species. Addition of TEMPO resulted in partial decomposition of the boron species but no indication that the reaction proceeds via a radical pathway. Detailed mechanistic studies I’m sure will be forthcoming.
As they comment in the paper, the clear advantages here over established methodology are that this protocol does not require flammable hydrogen gas, high-pressure equipment, or metal catalysts.
Engineered nitro-reductases have also been used in reduction of aryl nitro compounds. A recent example from Bornadel in OPRD describes a combination of nitroreductase enzymes and vanadium pentoxide.13Another couple of things I didn’t know- Vasicine (Figure 1), a readily available quinazoline alkaloid from the leaves of Adhatoda vasica, enables an efficient metal- and base-free reduction of nitroarenes to the corresponding anilines in water.14 D-Glucose in DMSO/water can also be used as a hydrogen source for aryl nitro group reduction.15
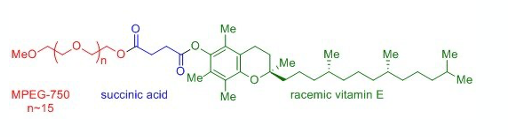
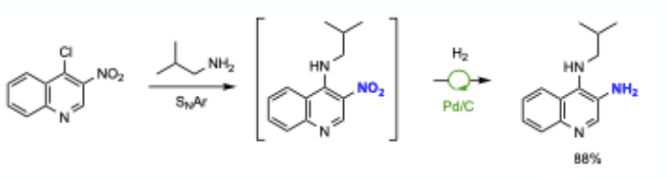
A second new paper focusing on aryl nitro group reduction returns to the traditional Pd/C hydrogenation approach. Bruce Lipshutz in collaboration with Novartis has published work on the use of low loadings of commercial Pd/C in water with either hydrogen gas or transfer hydrogenation using a silane.16 2 wt% of the commercially available surfactant TPGS-750-M (Figure 2) is required and the paper describes the reduction of nitro-containing important pharmaceutical intermediates. Several one-pot procedures including SNAr substitution followed by nitro reduction are described (Scheme 1).
A second new paper focusing on aryl nitro group reduction returns to the traditional Pd/C hydrogenation approach. Bruce Lipshutz in collaboration with Novartis has published work on the use of low loadings of commercial Pd/C in water with either hydrogen gas or transfer hydrogenation using a silane.16 2 wt% of the commercially available surfactant TPGS-750-M (Figure 2) is required and the paper describes the reduction of nitro-containing important pharmaceutical intermediates. Several one-pot procedures including SNAr substitution followed by nitro reduction are described (Scheme 1).
Some interesting developments in the nitro-reduction world here. Organocatalytic approaches in particular are a welcome addition to existing methods.
Scientific update run an online short-course on organocatalysis that might be of interest. Check our website (www.scientificupdate.com) or drop me an email ([email protected])
See you next time.
References:
- To view and download slides from a recent webinar I gave on modern nitration chemistry follow this link too the webinar archive on our website: https://www.scientificupdate.com/webinar_events/modern-nitration-chemistry/20210407/
- Recent developments in the reduction of aromatic and aliphatic nitro compounds to amines, M. Benaglia et al, Org. Process Res. Dev. 2018, 22, 430-445.
- Béchamp Reduction. In Comprehensive Organic Name Reactions and Reagents, Z. Wang (Ed) 2010, https://doi.org/10.1002/9780470638859.conrr063
- Dyes in the pharmaceutical industrty, M. Wainwright Dyes and Pigments 2008, 76, 582-589 (free to access).
- Johann Peter Griess FRS (1829–88): Victorian brewer and synthetic dye chemist: https://doi.org/10.1098/rsnr.2015.0020
- Recent trends in the chemistry of Sandmeyer reaction: a review, R. Akhtar et al, Mol Divers2021: https://doi.org/10.1007/s11030-021-10295-3
- Scale-up and safety evaluation of a Sandmeyer reaction, T. Pittelkow et al, Process Res Dev. 2004, 8, 1059−1064.
- a) Metal-free reduction of aromatic nitro compounds to aromatic amines with B2pin2 in isopropanol, Y. Wu et al, Org. Lett. 2016, 18, 2774-2776; b) Metal-free reduction of nitro aromatics to amines with B2(OH)4/H2O, H. Zhou et al, Synlett 2018, 29, 1765-1768
- A mild, DNA-compatible nitro reduction using B2(OH)4, N. Simmons et al, Org. Lett. 2019, 21, 2194-2199.
- 4,4′-Bipyridyl-catalyzed reduction of nitroarenes by bis(neopentylglycolato)diboron, K. Mashima et al, Org. Lett. 2019, 21, 9812-9817.
- Metal-free, rapid, and highly chemoselective reduction of aromatic nitro compounds at room temperature, M. Han et al, J. Org. Chem. 2022, 87, 910-919.
- Potential safety hazards associated with using N,N‑dimethylformamide in chemical reactions Q. Yang et al, Org. Process Res. Dev. 2020, 24, 1586-1601.
- Process development and protein engineering enhanced nitroreductase-catalyzed reduction of 2-methyl-5-nitro-pyridine, A. Bornadel et al, Process Res. Dev. 2021, 25, 648–653
- Metal-free transfer hydrogenation of nitroarenes in water with vasicine: revelation of organocatalytic facet of an abundant alkaloid, N. Kumar et al, J. Org. Chem. 2014, 79, 9433-9439.
- Catalyst-free water mediated reduction of nitroarenes using glucose as a hydrogen source, N. Kumar et al, RSC Adv. 2013, 3, 4894-4898.
- High turnover Pd/C catalyst for nitro group reductions in water. One-pot sequences and syntheses of pharmaceutical intermediates, B. Lipshutz et al, Org. Lett. 2021, 23, 8114-8118.








