A highly diastereoselective chloride-ion driven dynamic kinetic resolution of a phosphinate ester- a key step in the synthesis of Fosdevirine
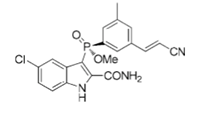
Fosdevirine (or GSK2248761A, top figure) is a non-nucleoside reverse transcriptase inhibitor, originally discovered by Idenix pharmaceuticals for the treatment of HIV infection. The structure is somewhat unusual in that it contains a phosphorus stereogenic centre- the required R-enantiomer is almost 23 fold more active against the wildtype virus than the S-form.
The original route to the chiral phosphinate intermediate involved late-stage classical resolution of the 2-indolecarboxylic acid using cinchonidine (see Ironmonger etal Org. Process. Res. Dev. 2018, 22, 200 for details of the synthesis and resolution).
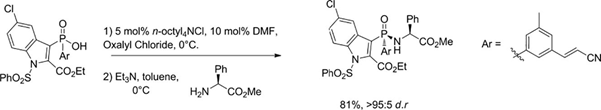
A recent follow-up publication by the team (Ironmonger etal Tett. Lett. 2018, 59, 2154) describes a highly efficient dynamic kinetic resolution of the glycinamide during the reaction of phosphinyl chloride with methyl-(S)-phenylglycinate. Several chiral amines were screened, however this gave the best selectivity. The chiral intermediate was isolated in 81% yield and d.r. >95:5 on a 10g scale.
Mechanistically the intermediate phosphinyl chloride is rapidly racemized by attack of chloride ion at phosphorus.[1] One enantiomer selectively reacts with the glycinate giving glycinamide, the remaining phosphinyl chloride is racemized. Thus process drives all of the phosphinyl chloride through to the required diastereoisomer. The chiral auxiliary is removed by heating in a methanolic solution of methanesulfonic acid at 50°C for 2 hrs. The chiral integrity of the P-stereogenic centre remains intact.
[1] For a review on nucleophilic substitution at phosphorus: stereochemistry and mechanism see Tett. Assy. 2017, 28, 1651








