The direct halogenation of phenols using electrophilic halogenating reagents such as NBS are frequently used to prepare halogenated phenols. The problem with this method is that selectivity can often be low- the ortho– isomer is usually the minor component in a statistical mixture due to steric and electronic effects, and over reaction to give polyhalogenated material is common. A direct method to prepare ortho-substituted phenols cleanly and in high yield is attractive to synthetic chemists. The halogenated products are useful intermediates for synthesis of fine chemicals and pharmaceutical intermediates in addition to BINOL-type ligand precursors.[1]
Traditional methods to overcome the inherent lack of selectivity involve directed ortho-metalation or, to a lesser extent, transition-catalyzed approaches. Both suffer from drawbacks and have limited substrate scope. Amine catalysed ortho- bromination with NBS reported by Fujisaki and later publications by Maddox on catalytic bis-thiourea chlorination using NCS suggested that catalytic approaches might be the way forward to improve efficiency, selectivity and practicality for large scale synthesis.[2]
A paper published earlier this year by Yeung (ACS Catal. 2018, 8, 4033) builds on this early work and describes in detail an ammonium salt-catalyzed process using 1,3-dichloro-5,5-dimethylhydantoin (DCDMH) in toluene as the stochiometric chlorinating agent. Ortho- selective selenylation is also discussed.
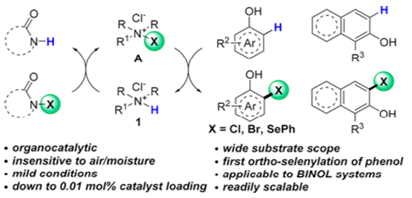
A range of salts (1 in scheme) were investigated and (1, R=iPr, R1=H) gave the highest selectivity, with a catalyst loading of 1mol%. The N-halo intermediate (A in the scheme) is thought to be the active species and was consistent with NMR shift experiments. Interestingly the chloride ion appears to play a role in ortho- site selectivity (bromide and acetate were inferior). It’s believed this is related to the chloride salt being a better hydrogen bond acceptor to interact with the phenolic proton.
Functional group tolerability was very good and included boronic esters, aldehydes/ketones, carboxylic esters and even a steroid (estrone) nucleus. The chemistry is exemplified by a wide range of phenol substrates and demonstrated on gram-scale. In addition a number of symmetrical and unsymmetrical BINOL ligands were halogenated regioselectively and the chloro-aryl products elaborated by Pd catalysed cross-coupling chemistry.
The chemistry remains to be investigated at scale, in particular the thermal safety of the intermediate N-halo species, but it looks to be a good practical method to effect the required transformation.
[1] For a review of Halogenation of organic compounds using continuous flow and microreactor technology see React. Chem. Eng. 2017, 2, 7-19; Use of bromine and bromo-organic compounds in organic synthesis Chem. Rev. 2016, 116(12), 6837-7042
[2] Bull. Chem. Soc. Jpn. 1993, 66, 1576; Org. Lett. 2016, 18(21), 5476








