A number of years ago, during a scale-up campaign, I worked to try and convert a relatively simple, high-yielding Suzuki coupling from a relatively standard set of homogeneous coupling conditions to heterogeneous conditions using a palladium on carbon (Pd/C) catalyst. The reason for making the change was that although the homogeneous conditions worked very well, the subsequent work-up to remove the residual Pd from the reaction product was lengthy and complex, and only expected to become more challenging as we scaled-up from the R&D lab to the kilo lab. In this case we were able to get the heterogeneous reaction to work and there were negligible quantities of Pd in the isolated product after filtering off the catalyst, but the yield was significantly lower and so we stuck with homogeneous conditions for the next campaign. Unfortunately the project was discontinued before we could perform more detailed optimization on the heterogeneous conditions, however this whole experience left me with many questions about how much I really understood about the mechanisms of Pd-mediated reactions. Why did some forms of Pd/C work better than others? Is the reaction taking place on the catalyst surface or in solution?
Many teams have tried to answer these questions over many years based on circumstantial evidence but a recent paper by the Ananikov team based at the Russian Academy of Science in Moscow (J. Am. Chem. Soc. 2023, 145, p 9092) provides direct visual evidence of what is taking place. Previous studies have used electron microscopy to visualize the catalyst surface before and after the reaction, but typically random areas are visualized. The breakthrough described in this paper is that by using machine learning image recognition software the authors were able to reliably visualize exactly the same spacial region of the catalyst on a nanometer scale before and after the reaction. They achieved this by identifying features of the catalyst support starting at mm scale then stepwise zooming in over six iterations of increasing magnification as shown in the images below. The sequence of images zooming in from the mm scale to the nm scale reminded me starting with Google or Apple Maps fully zoomed out so that it shows the globe, then zooming all the way in to see your own house in detail.
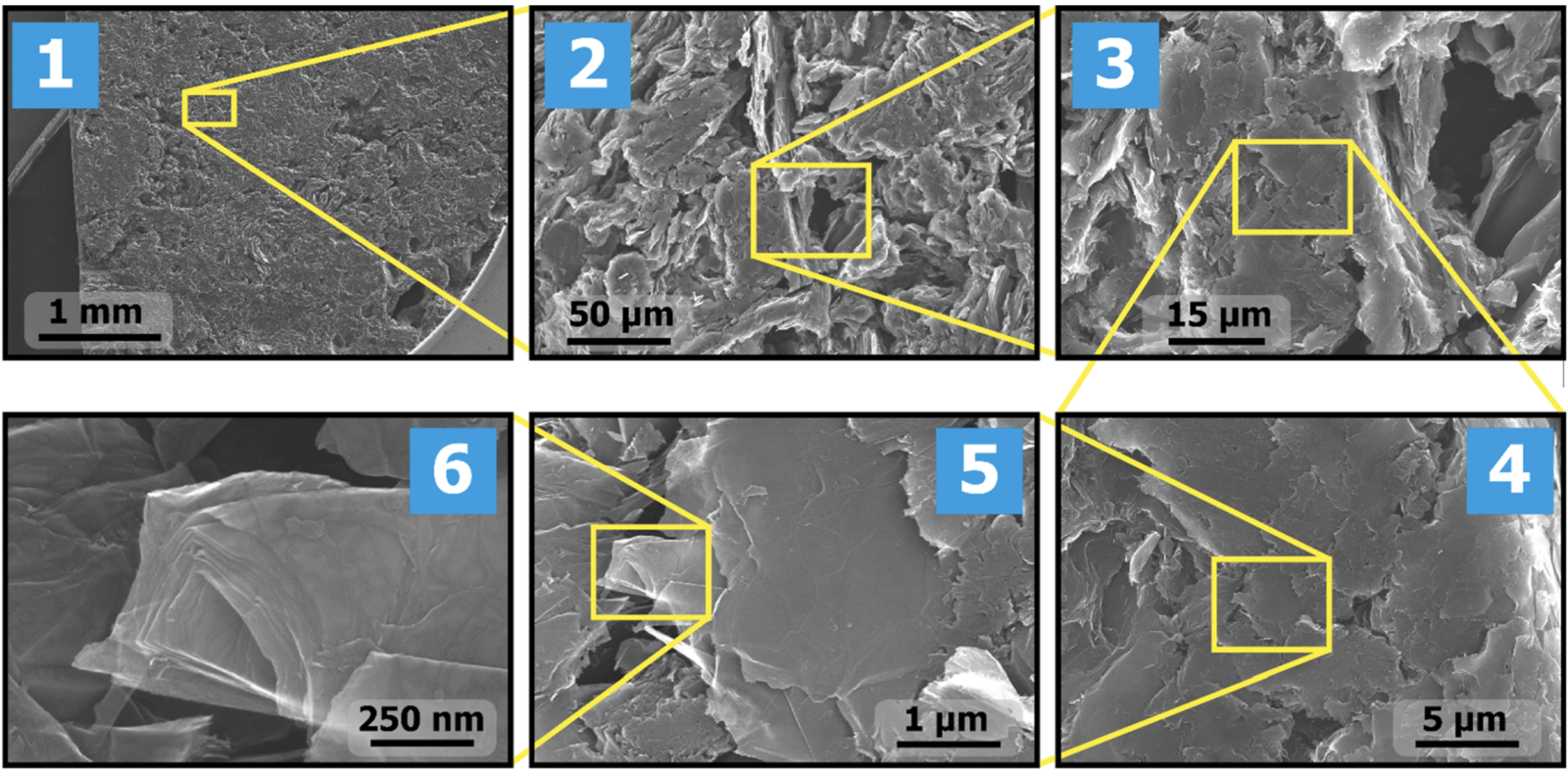
Previous attempts to do this had failed due to the difficulty of imaging the catalyst in exactly the same way before and after the reaction, but the ML algorithm was able to identify landmark geometrical features regardless of exactly how the substrate was presented to the detector. Previous studies had relied on random sampling of the surface so it was not possible to definitively conclude which changes had occurred during the operation of the catalytic system, and which were due to variations across different regions of the catalyst surface.
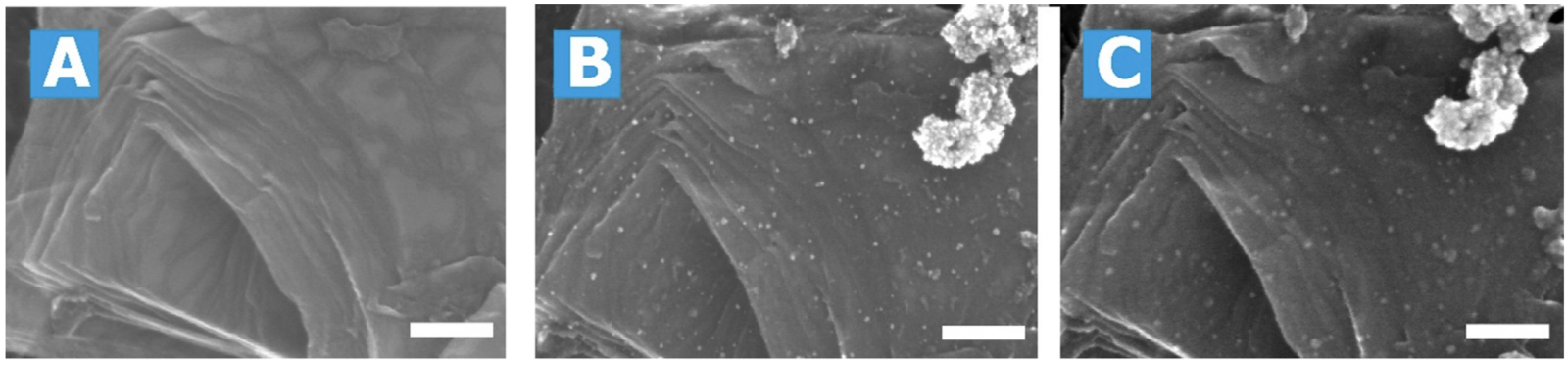
Once zoomed in the images are able to show individual Pd atoms and morphological features of Pd-nanoparticles on the substrate surface, and track their changes in position, size and shape before and after the reaction. The three images below show the substrate before catalyst deposition (A), the Pd/C catalyst before (B) and after (C) the reaction. By further using image recognition software the authors were able to quantify the changes in location and size of the particles between pairs of images, such as B and C.
In the study the authors explored two reactions:
- A Mizoroki-Heck reaction performed in DMF with Et3N at 140 C
- A Suzuki-Miyaura reaction performed in EtOH/H2O (4:1) with K2CO3 at 70 C
In both cases the Pd/C catalyst was generated by gradual decomposition of Pd2(dba)3 which is known to generate a range of different Pd centers and nanoparticles on the C surface, and which the authors had previously shown to be active in these reactions, as well as transfer hydrogenation and debenzylation reactions.
Of particular interest to me was a type of cross-over experiment where:
- An initial experiment was started with 1 mol % Pd loading relative to the substrates and heated up for only 10 min under the reaction conditions above. As might be expected no conversion was observed in such a short period
- The supported Pd/C was then removed and transferred (reloaded) to an identical reaction mixture
- Both the supernatant from the initial experiment and the reloaded reaction mixture were heated further
Remarkably in both cases it was the reaction with only the supernatant that proceeded further despite ICP-OES measurements showing that at least 99.5% of the Pd remaining on the catalyst surface. For the Mizoroki-Heck this corresponded to 4.5 x 10-3 mol % giving 54% conversion after 8 h, and for the Suzuki-Miyaura 100% conversion was achieved in 1 h using only 5.0 x 10-3 mol % Pd! Further analysis showed that the species in the supernatant were individual Pd atoms, not nano clusters. This conclusion was supported by the images that show that the mechanism for catalyst deactivation was trapping of the single atom catalysts by the metal nano particles rather than by the carbon surface: i.e. nanoparticles act as poisons of single-atom catalytic centers.
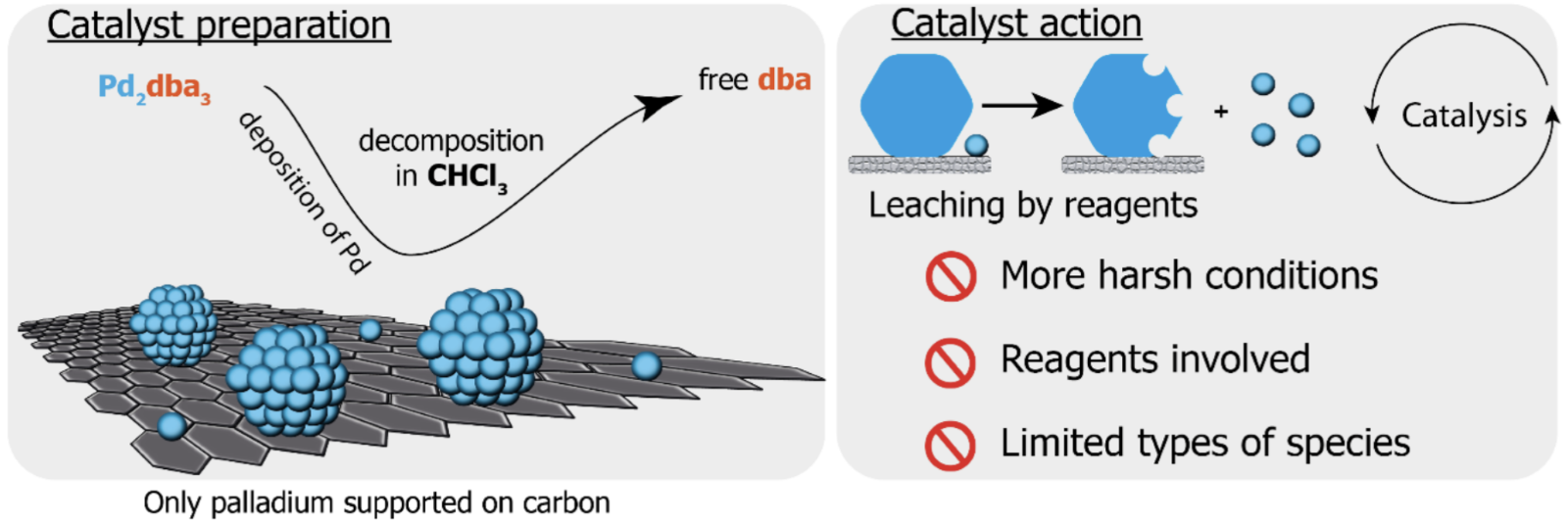
In a recent follow-up paper (Inorganics 2023, 11 (6), 260) the same group of authors have further characterized the species formed by the decomposition of Pd2(dba)3 and how they are deposited on the carbon surface. This team has published numerous papers in recent years and has introduced the concept of ‘cocktail catalysis’: i.e. that particles can leach from the surface of the support to form a dynamic system consisting of multiple species, each of which will have different catalytic activity. The previous understanding, shown immediately below, was that the Pd/C catalyst consisted of only metal particles (nanoparticles, clusters and single atoms)
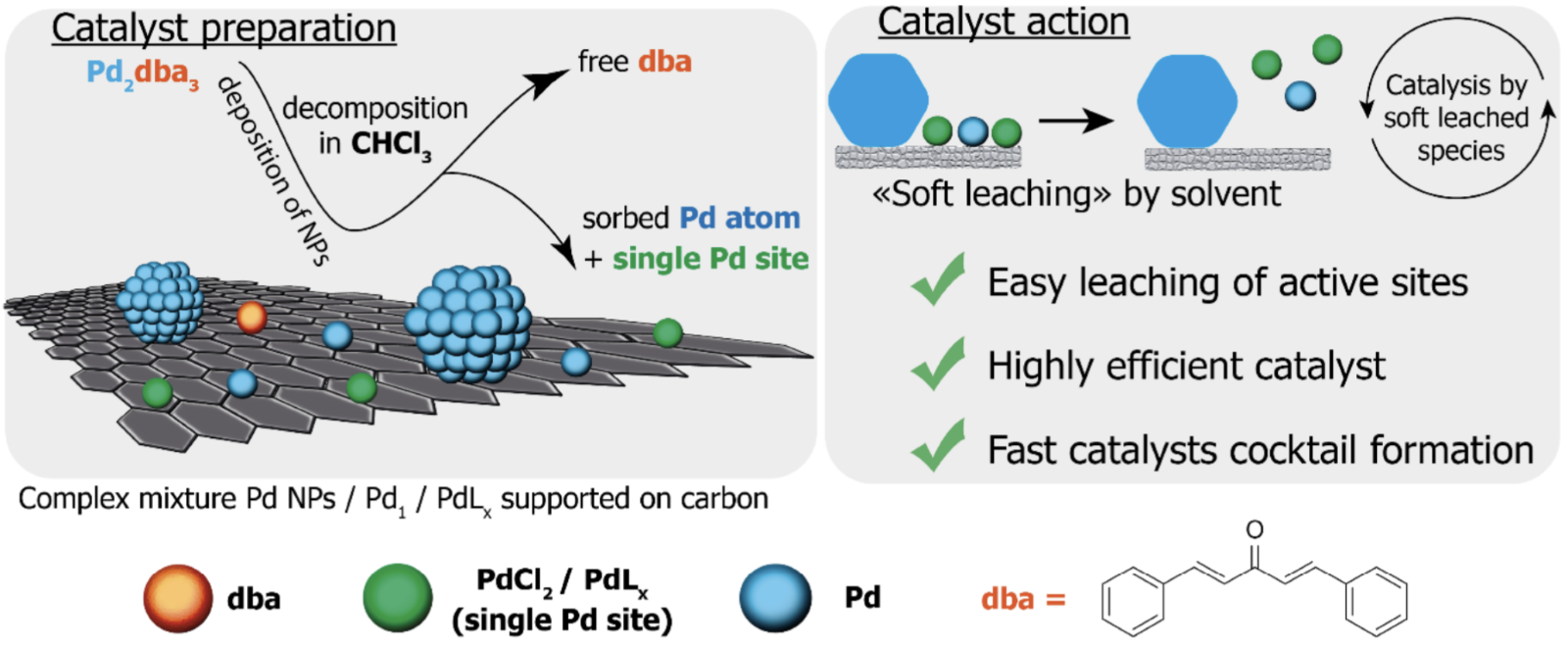
The data from this most recent study show that the picture is much richer than previously assumed and that a number of highly active “hidden” metal centers are formed on the carbon surface as illustrated below. These highly active species are also more readily leached into solution which explains why the reaction with the supernatant proceeds further than the reloaded reaction despite containing 0.5% or less of the total Pd.
More complex range of species now known to be present
As the authors state in the JACS paper: “Tracing the fate of individual nanoparticles and individual atoms of a catalyst in the reaction can open up unthoughtful ways to understand and develop catalysis.” Hopefully these imaging tools will be broadly adopted by the catalysis community seeking to develop new catalysts and reaction conditions for metal-mediated process that will be adopted by the synthetic chemistry community. As we better understand the true nature of the active metal species in the reaction mixture we might be able to significantly improve the sustainability of these reaction types by first reducing the amount of metal needed to perform the reaction and then reducing the material needed to reduce the residual metal level to acceptable levels.








