The complex machinery required to take a drug through development and regulatory approval can be as arduous as the discovery of the molecule itself. In recent times the demand for COVID-19 drugs has added to the pressure to expedite this process in record time and several organizations working in the small molecule arena have met this challenge, notably Merck with their nucleoside derivative Molnupiravir that targets viral replication by promoting mutations in viral RNA,1a and Pfizer with Nirmatrelivir- a peptidomimetic that inhibits the viral MPRO main protease, again targeting viral replication.1b The latter is co-administered with the HIV drug Ritonavir. This additive increases blood concentrations of Nirmatrelivir by inhibiting its major metabolising P450 enzyme Cyp 3A4. Ritonavir (or Norvir to give its original Abbott brand name) has an interesting history that I’m sure many will be familiar with. If not, google ‘Ritonavir Polymorph’ and see where it takes you.2 Pharma companies have, however, in many ways become victims of their own success- with business leaders saying if you can do it here why can’t we make this the new norm? Speaking as a process chemist I know this not a sustainable business model. A debate for another time perhaps.
The global effort to discover and develop small molecule COVID-19 antivirals, targeting novel mechanisms of action, is still very active, despite the World Health Organisation’s statement that the COVID pandemic no longer constitutes a public health emergency of international concern.3
Another drug developed to inhibit the viral 3CL protease is Ensitrelvir (S-217622, Figure 1).4 Discovered by Shionogi through a research collaboration with Hokkaido university, its approved and marketed in Japan under the brand name Xocova.
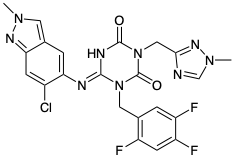
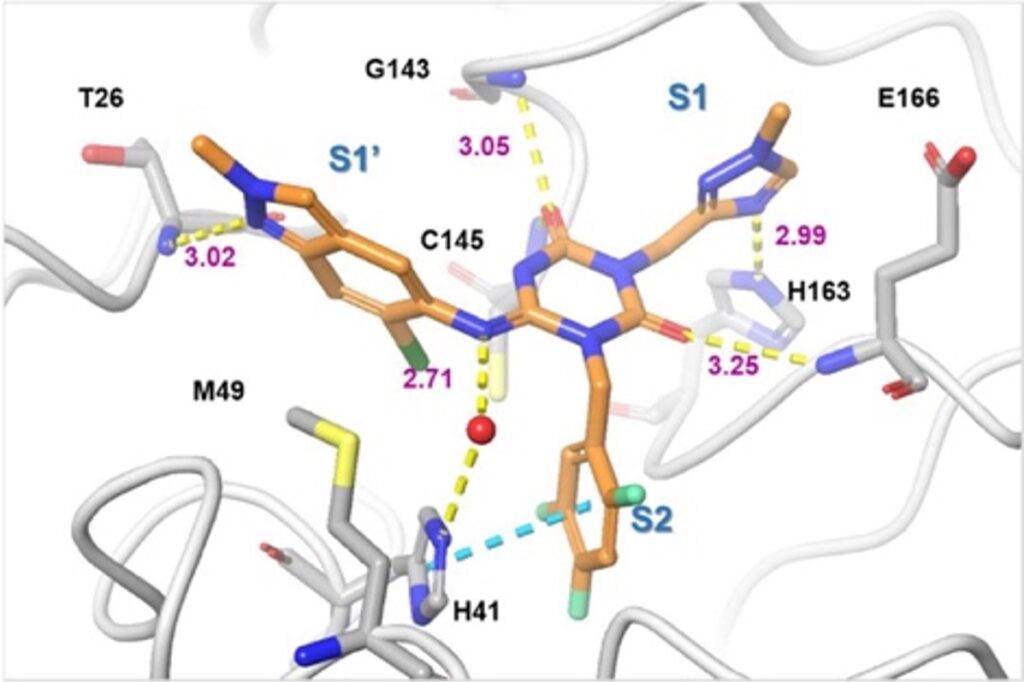
Figure 2 shows the X-ray co-crystal structure of 3CLPRO complexed with Ensitrelvir.6 In the S1 site, the 1-methyl-1H-1,2,4-triazole unit binds into the S1 pocket, forming a hydrogen bond with the side-chain NH of His163. The 2,4,5-trifluorobenzylic moiety occupies the hydrophobic S2 pocket and stacks with the side chain of His41. The P1’ ligand, 6-chloro-2-methyl-2H- indazole moiety hydrogen bonds with the Thr26 main-chain NH and hydrophobic contact with Met49.6 If MedChem isn’t your thing lets move on to the more interesting synthetic aspects.
A paper published earlier this year by Shionogi in ACS Central Science describes development of a manufacturing route to Ensitrelvir.4 This is a follow-up to their paper from 2022 describing synthesis of the key triazone-triazole intermediate.5
The process used to make the API is shown in Figure 3.4 One aspect of the synthesis that I found particularly interesting was the introduction of a phenol-fragment (Figure 3, highlighted in red) to address a number of challenges that became apparent during process development. Remarkably that single structural fragment:
- Serves as a protecting group.
- Stabilises a key intermediate-shielding it from competing hydrolysis during work-up.
- Traps a reactive carbocation formed during a tert-butyl deprotection reaction.
- Functions as an atypical leaving group in the SNAr chloro-indazole coupling in the final API step (Figure 3).
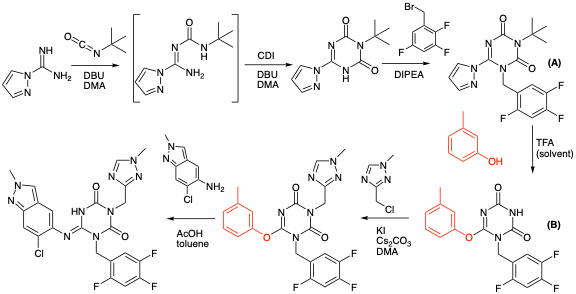
Lets go through each of these points in more detail. The fist problem encountered during the synthesis was cleavage of the tert-butyl protecting group at the 3-position of the pyrazolyl intermediate (A, Figure 3). As it turned out this was extremely challenging. Protic and Lewis acids all proved to be ineffective (MeSO3H, H2SO4, HCl, BF3Et2O etc.). In fact the only conditions that were suitable required using TFA as both a reagent and solvent. Working up reactions involving large volumes of TFA is not easy to do. In the lab we would most likely just distil off the TFA under reduced pressure and chase-out the last traces using a higher boiling solvent such as toluene. On large scale this is not practical. TFA is extremely corrosive and notoriously difficult to handle (I speak from experience here), so neutralization is the preferred method of dispatch. This is where things started to go wrong. The pyrazolyl moiety in intermediate (A)- or more precisely the product from the tert-butyl deprotection- proved to be unstable to the neutralization conditions- both in aqueous acid or base- particularly the latter. Figure 4 shows a plot of the stability of a structurally related intermediate with both the pyrazole and 3-methylphenoxy groups at the 6-position (acidic conditions: 1.5 eqv. AcOH in DMA/H2O, basic conditions: 1.5 eqv DIPEA, DMA/H2O). At pH4, recovery of the 6-pyrazoly derivative after 24hrs was around 50%. At pH 11 very rapid hydrolysis was observed- with virtually nothing left after 8 hours (Figure 4). So in other words work-up under acidic or basic conditions, and exposure of a 6-pyrazolyl group to extended processing times, would result in almost complete destruction of the product. Extended processing times are an inevitable factor in running large scale reactions and work-ups so this was very bad news.
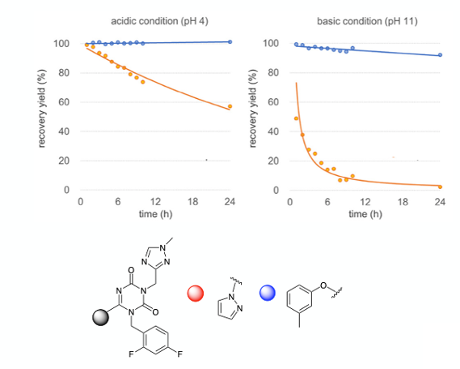
Reading between the lines a lot of work was carried out to find an alternative to the 6-pyrazole that would serve the same purpose but be less susceptible to hydrolysis. And this is where meta-cresol enters the picture. Addition of meta-cresol to the TFA deprotection reaction resulted in simultaneous deprotection of the tert-butyl group at the 3-position and protection/activation of the 6-position to give (B, Figure 3).4 Using an excess amount of meta-cresol also played a role in trapping the tert-butyl cation generated during the TFA deprotection, preventing formation of alkylated impurities originating from prolonged contact of the product with this reactive intermediate.7 The supplementary information gives experimental details for preparation of intermediate (B) on 183 Kg scale (!) (using 105 Kg, 2 eqv., of meta-cresol) in 91% isolated yield.8
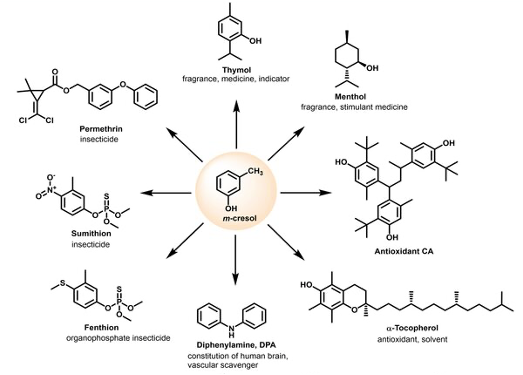
Meta-cresol is an inexpensive commodity material made industrially from fossil sourses.9a I looked up a few interesting facts. It was apparently first discovered in cow’s urine in 1851 by Stadeler.10 Not a place one would want to spend too much time foraging. Soon after, in 1854, it was discovered in coal-tar by Fairlie and Williamson and is a component of creosote- a high boiling fraction obtained from coal-tar distillation. Cresols are also common in Nature. They are formed by metabolic processes in various microorganisms and are present in urine from mammals. Apparently, humans excrete around 90mg of para-cresol per day in urine. Who knew. Meta-cresol has also been used as a preservative for insulin preparations. A major application is in the fine chemical and flavours industry. Meta-cresol is a synthetic precursor to Tocopherol (vitamin E), thymol, menthol, the insecticides Fenthion, Sumithion and Permethrin and various resin plasticizers (Figure 5).9b
Back to the paper. Intermediate (B) had much higher stability in the work-up of the TFA deprotection (Figure 3). Aqueous sodium acetate was used to neutralize the acid and the product crystalised directly from the reaction mixture with no extractions required. A process chemists dream. But nothings ever simple. Product (B) crystallised as a co-crystal with meta-cresol. Not necessarily a bad thing- co-crystals can be used to purify materials and give improved solid form characteristics.11 In fact meta-cresol can be separated from the very close boiling para-isomer by co-crystal formation with phenol.11b However in our case it had a detrimental effect on the penultimate alkylation step (Figure 3). After much investigation an extractive work-up was developed and proved to be the way forward. Neutralization was carried out using 9% aq. NaOH (in the presence of ethyl acetate as solvent), adjusting the pH to 6, and the compound subsequently crystallised (after seeding) from EtOAc using heptane as an antisolvent.8 I can only imagine how long it took to neutralise that volume of TFA (682 Kg to be precise).
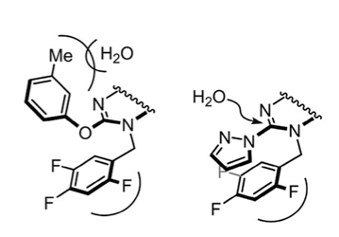
So we already have two positive effects imparted by meta-cresol: stability with respect to a basic (NaOH) work-and scavenging the carbocation formed during the tert-butyl group cleavage. Anything else? Well yes as it happens. Introduction of the meta-cresol moiety had a pivotal impact on the subsequent alkylation reaction arising through the increased stability of (B) under acid and basic conditions. Figure 4 shows the remarkable increase in stability of the phenol-derived intermediate relative to the corresponding pyrazole. In the paper they comment that its most likely due to a combination of electronic and steric effects (Figure 6). To me that’s code for “we don’t really know but a reviewer wanted us to add a comment- or even better a picture” No mention of the Burgi-Dunitz and/or Flippin-Lodge angle. I might be being overly cynical here.
And then finally meta-cresol- and phenoxides in general- are atypical leaving group for SNAr type reactions.12 Probably why the phenoxypyrimidine core is sufficiently unreactive to be used in in a number of bioactive molecules.13 However in this particular case reaction with the chloro-indazole amine (Figure 3, AcOH / toluene, 100°C) worked extremely well. In fact it turned out to be superior to other activating substituents such as ethansulfanyl (used in the original discovery chemistry route) and of course the original pyrazoyl system referred to above.6 Several patents describing alternative synthetic approaches have been filed.14
I think you would be hard pushed to find another substituent that contributes so much to the robustness of an API manufacturing route. Congratulation to the Shionogi team.
See you next time.
References:
- a) Development of A robust manufacturing route for Molnupiravir, an antiviral for the treatment of covid-19: P. Fier et al, Org. Process Res. Dev. 2021, 25, 2806–2815; b) Development of the commercial manufacturing process for Nirmatrelvir in 17 months: J. Ragan et al, ACS Cent. Sci. 2023, 9, 849–857
- Dealing with the impact of ritonavir polymorphs on the late stages of bulk drug process development: S. Chemburkar et al, Org. Proc. Res. Dev. 2000, 4, 413–417; Disappearing polymorphs revisited J. Bernstein et al, Angew. Chem. Int. Ed. 2015, 54, 6972-6993
- https://covid19.who.int
- Development of a manufacturing process toward the convergent synthesis of the covid-19 antiviral Ensitrelvir: T. Kawajiri et al, ACS Cent. Sci. 2023, 9, 836−843
- Two-stage one-pot synthetic strategy for the key triazone-triazole intermediate of Ensitrelvir (S-217622), an oral clinical candidate for treating COVID-19: W. Chen et al, RSC Adv., 2022, 12, 34808-34814
- Discovery of S-217622, a noncovalent oral SARS-CoV-2 3CL protease inhibitor clinical candidate for treating COVID-19: Y. Tachibana et al, J. Med. Chem. 2022, 65, 6499-6512
- Contemporary carbocation chemistry: applications in organic synthesis: D. Klumpp et al, Chem. Rev.2013, 113, 6905-6948
- https://pubs.acs.org/doi/suppl/10.1021/acscentsci.2c01203/suppl_file/oc2c01203_si_001.pdf
- a) Cresols and xylenols: H. Fiege et al, Ullmans Encyclopaedia of Industrial Chemistry, Wiley 2012, 10, 419-461; b) De novo biosynthesis and gram‑level production of m‑cresol in Aspergillus Nidulans: W. Wang et al, Appl. Microbiol. Biotechnol. 2021, 105, 6333-6343
- Stadeler, Ann. Chem. Pharm. 1851, 77, 17 -37
- a) Screening and preparation of co-crystals: a comparative study of mechanochemistry vs slurry methods: M. Zarworotko et al, Cryst. Growth Des. 2021, 21, 4141-4150; b) Separation of meta-cresol from meta cresol-para cresol mixture: US2042331A
- Phenoxide leaving group SNAr strategy for the facile preparation of 7-amino-3-aryl pyrazolo[1,5-a]pyrimidines from a 3-bromo-7-phenoxypyrazolo[1,5-a]pyrimidine intermediate: J. Catalano et al, Tet. Lett. 2015, 56, 6077-6079
- Copper-catalyzed direct syntheses of phenoxypyrimidines from chloropyrimidines and arylboronic acids: a cascade avenue and unconventional substrate pairs: P. Gogoi et al, Org. Chem. 2022, 87, 11846−11851 (paper introduction)
- Preparation method of novel coronavirus main protease inhibitor: 2022, CN114853741; Green preparation of ensitrelvir for treating coronary pneumonia: 2022, CN114751894








