The importance of methylation in the discovery and development of bio-active small molecules is well known. I presented a webinar a couple of years ago on the “The magic methyl in medicinal chemistry,” demonstrating the unique properties of this, the simplest functional group.1 I also reviewed the state of the art in synthetic approaches to C-H methylation. Not surprisingly, new mythology continues to be introduced, with green and metal free processes being favoured, and safer (from a toxicity perspective) replacements for simple halide- alkylating agents a significant selling point.
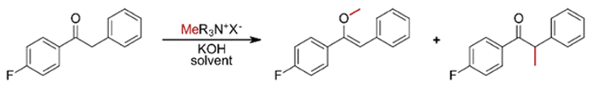
A recent paper describes a methylation approach that is not new, but which has remained on the periphery of useful synthetic methodology for some time. Quaternary ammonium salts are generally utilized as phase transfer catalysts or ionic liquids. Less commonly they have been used as alkylating agents, particularly for benzyl-group transfer. Impurities in phase transfer catalysed reactions are sometimes related to alkyl transfer from the quaternary nitrogen to an opportunistic nucleophile. Direct methylation of oxygen (e.g. a phenol),2a nitrogen (e.g. N-methylation of an amide)2b or carbon (via a C-H activation process)2c nucleophiles has been described in the literature but remains underexploited, particularly for methylation. Schnurch has stoked renewed interest in this area, describing the use of phenyltrimethylammonium iodide as a non-volatile, non-carcinogenic methylating agent for introducing a CH3 group into the α-position of a carbonyl group.3
Using benzyl 4-fluorophenyl ketone as a test substrate, various ammonium salts were initially screened for conversion to either the C- or the O- methylated product (Figure 1). The reaction mixtures were analysed via 19F NMR using trifluoro toluene as an internal standard to enable rapid analysis and optimisation. Phenyltrimethylammonium iodide appeared superior to the tetramethylammonium salt, presumably due to activation of the nitrogen atomin the former via electron withdrawing effects. The by-product from the methylation reaction is N,N-dimethylaniline- a toxic compound that a harsh critic might claim is worse that using methyl iodide. This might well be a moot point. No di-methylated products were ever observed in the experiments. Several solvents were investigated- with anisole being the preferred system, both in terms of conversion and increased levels of C-alkylation over vinyl ether fomation. KOH was the preferred base compared with other metal salts (including NaOH, Cs2CO3, KOtBu).
Since the O-methylated vinyl ether could theoretically act as a methylating agent for C-methylation, a number of control experiments were carried out. Both products were found to form simultaneously, and prolonged heating of the reaction mixture did not change the product ratio. To exclude the possibility that thermal decomposition of the ammonium salt might generate methyl iodide (a possible methylation mechanism) experiments were run in a two-chamber (Skrydstrup) reactor containing anisole/ammonium salt and base at 130°C in one chamber and a gas-phase connector to the second chamber containing the ketone substrate.4 No methylated products were detected in this experiment. This result is somewhat surprising based on the recent mechanistic work by Reid et al that suggests that in situ generated methyl iodide is likely to be the main methylating reagent in these reactions.5 Reid comments that “carefully selected anilinium iodide salts could function as a safer, crystalline, and storable ‘slow bleed’ alternative to using carcinogenic and low- boiling methyl iodide directly.” More experimental work required here I think.
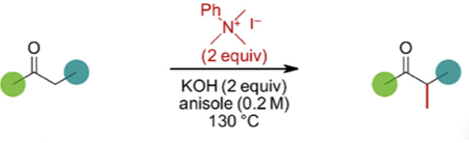
Several structural variations of the core benzyl ketone were investigated together with a few other ketone substrates. Yields were moderate to good (Figure 2). Significantly lower yields were observed using sterically hindered substrates and interestingly substrates that were less susceptible to enolization, e.g., 4-phenylcyclohexanone, resulted in diminished yields and mostly recovered starting material (17% v’s 78% for benzyl 4-fluorophenyl ketone).
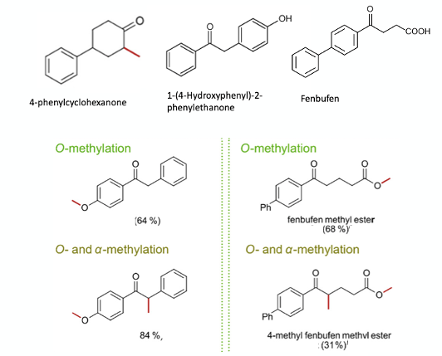
Using 1-(4-hydroxyphenyl)-2-phenylethanone as substrate, methylation initially occurred at the phenolic oxygen, and subsequently at the α-position of the carbonyl. Reaction of the nonsteroidal anti-inflammatory drug fenbufen resulted in initial methylation of the carboxylic acid moiety. Upon addition of fresh reagent and further heating further methylation at the α-position (Figure 3).
Introduction of larger alkyl substrates- α-Ethylation (with PhEt3NI) and α-benzylation (using BnMe3NCl) also appeared to work. In the latter case, not surprisingly, the benzyl group transferred preferentially over a methyl group.
A bit more work to do to make this a go-to method for methylation, however this paper serves to stimulate interest in this approach. As an aside- a useful paper by Richard Fox published a couple of years ago in OPRD describes partitioning of tetraalkylammonium salts in organic solvent/water mixtures.6 Very useful if removal of the salt is important for your process.
Oh- and by the way, fans of 90’s TV sitcom Frasier might recognise the title of this article as a quote from Niles in the episode “Dad loves Sherry, the boys just whine”
See you next time.
References:
- The magic methyl in medicinal chemistry, J. Studley, 2020, https://www.scientificupdate.com/webinar_events/the-magic-methyl-in-medicinal-chemistry/20200429/
- a) Synthesis of aryl alkyl ethers by alkylation of phenols with quaternary ammonium salts, N. Maras et al, Acta. Chim. Slov. 2010, 57, 29-36; b) Selective methylation of amides, N-heterocycles, thiols, and alcohols with tetramethylammonium fluoride, H. Cheng et al, Org. Lett. 2020, 22, 331-334; c) Phenyltrimethylammonium salts as methylation reagents in the nickel-catalyzed methylation of C-H bonds, T. Uemura et al, Angew. Chem., Int. Ed. 2016, 55, 3162− 3165.
- Selective α‐methylation of aryl ketones using quaternary ammonium salts as solid methylating agents, M. Schnurch et al, J. Org. Chem. 2022, 87, 4305−4315.
- The development and application of two-chamber reactors and carbon monoxide precursors for safe carbonylation reactions, Skrydstrup et al, Acc. Chem. Res. 2016, 49, 594– 605.
- Trialkylammonium salt degradation: implications for methylation and cross-coupling, M. Reid et al, Chem. Sci., 2021, 12, 6949-6963.
- General user guide for partitioning of tetraalkylammonium and tetraalkylphosphonium salts: impacts of cation, anion, and solvent, R. Fox et al, Org. Process Res. Dev. 2020, 24, 235–241.








