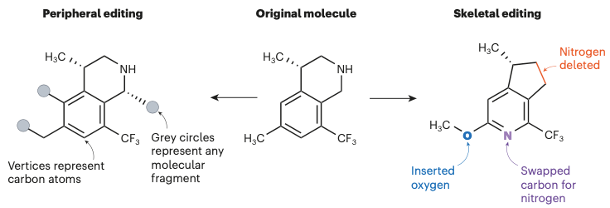
Single atom deletion and skeletal editing of complex organic molecules is an interesting and potentially very impactful area of research. Over 100 papers mentioning the term ‘skeletal editing’ have been published in the past 2 years. The ability to selectively add or remove a single heteroatom from a molecules core architecture is distinctly different from the peripheral modification of its structure- the mainstay of medicinal chemistry ligand optimisation. The concept is highlighted in figure 1. From a drug discovery perspective, the ability to completely alter the pharmacophore without having to resort to complex, multi-step synthesis, is a powerful method for the rapid exploration of chemical space and identification of new lead structures. There are plenty of examples in the literature of judicious incorporation of a heteroatom, for example phenyl to pyridyl, giving a boost in activity and/or metabolic stability.1
One of the first papers published in Nature Synthesis-a recent addition to the Nature family of journals- is a nice review on the subject by Mark Levin et al.2 A paper by Lu et al in Angew. Chemie. talks about N-atom deletion in nitrogen heterocycles via N-sulfonylazidonation and Curtius rearrangement.2b Morandi et al recently described the late-stage diversification of indole skeletons through nitrogen atom insertion.2c
The Levin group are very much in the vanguard of this field, and their recent paper in Science demonstrates the concept and the power of “structure editing” methodology- conversion of arly azides to pyridines.3
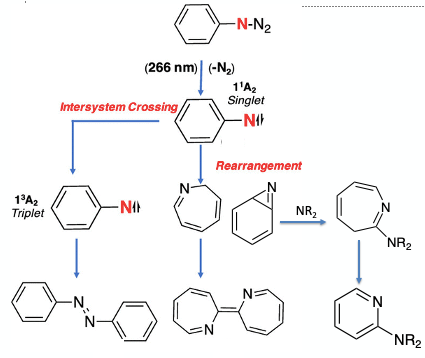
The process has its roots in seminal work published by Doering in 1996. Photolysis of phenyl azide in the presence of a nucleophile (diethylamine) generated 2-amino-3H-azepines via aryl nitrene insertion.4 The nature of the product obtained upon irradiation is dependent on the spin-state of the nitrene intermediate. Singlet phenylnitrene undergoes ring expansion to the azepine – an electrophile that can be trapped with a suitable amine nucleophile. Photo-irradiation followed by Intersystem crossing generates a triplet nitrene that dimerises to give azobenzene (Figure 2). Later work by Sundberg and more recently Burns showed that oxidation of 2-amino-3H-azepines with either triplet (ground state) or singlet oxygen results in para or meta deletion, skeletal rotation and amine retention (Figure 2).5
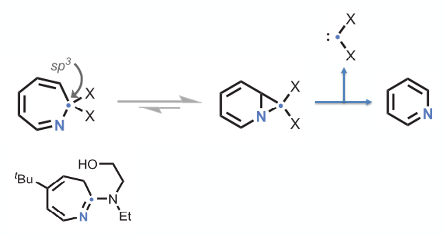
The key innovation in Levin’s science paper is ipso-deletion and conversion of an aryl ring to the corresponding pyridine. The unique design aspect is the incorporation of a pendent (hydroxy) group to the amine nucleophile to induce spirocyclisation and elimination of a carbene (Figure 3).
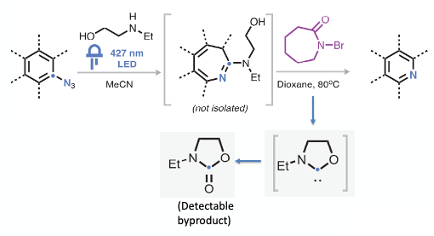
Irradiation of the appropriately substituted phenyl azide at 427nm in the presence of 2-(ethylamino) ethanol generated the aminoalcohol-substituted azepine derivative. Heating this material at 80°C with an oxidant ( N-bromocaprolactam) generated the pyridine. 4-Tert-butyl phenylazide gave a yield of 57% of the corresponding pyridine. A modest yield however the yield is arguably less important in a nitrogen screen where you are just concerned with making an analogue to screen.
Interestingly NBS didn’t work in the process and resulted in formation of thermally unstable succinimide addition products. N-bromocaprolactam is a weaker oxidant and seemed to do the trick. The by-product (N-ethyl oxazolidinone) was detected by NMR and mass spec. Control experiments demonstrated that the pendant alcohol was required for formation of the pyridine product.
In terms of the practicality of the method, a two stage one pot transformation (without isolation of the azipene) was adopted and substrate scope was demonstrated with plenty of examples in the paper (Figure 4).
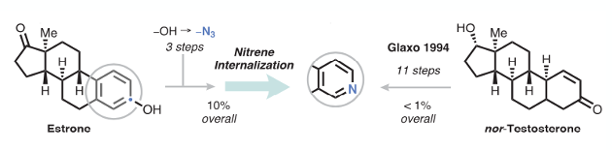
A complex (steroid) substrate example was used to demonstrate the potential application of the method for late-stage ring manipulation (Figure 5). Conversion of Estrone to nortestosterone- a process that typically takes over 10 synthetic steps giving the product in <1% yield-6 was achieved in 10% overall yield (including conversion of the esterone phenol to the azide in 3 steps) (Figure 5).
A recent paper by Burns et al describes conversion of aryl azides to aminopyridines,5 and another by Sarpong et al describes skeletal editing of pyrimidines to pyrazoles by formal carbon deletion.7
How are phenyl azides prepared?- carefully would be the cynical answer. Phenyl azide itself, prepared by the father of aryl diazotization-Peter Griess- in 1864, was the first organic azide to be prepared synthetically. General methods for the synthesis of aryl azides include diazotization of an aryl hydrazine, metathesis of an aryl iodide with sodium azide in the presence of Cu(I), or manipulation of the aniline using azide transfer reagents or hydazoic acid.8 A good summary of available methods, together with the safety aspects associated with the various approaches, is given in the introduction section of a paper by Richardson on the synthesis of 4-azido-L-phenylalanine.9 If you interested in azides and the safety of azides I would recommend our safety and selectivity training course.10
In summary- an interesting paper that demonstrates how a reaction found historically to be difficult to use synthetically can be tamed to enable medicinal chemistry nitrogen-screening.
See you next time.
References:
- The expanding role of pyridine and dihydropyridine scaffolds in drug design: Y. Wang et al, Drug Design, Development and Therapy, 2021, 15, 4289-4338
- a) Single-atom logic for heterocycle editing: M. Levin et al, Nat. Synth. 2022, 1, 352-364; b) N-atom deletion in nitrogen heterocycles: H. Lu et al, Angew. Chem. Int. Ed. 2021, 60, 20678-20863; c) Late-stage diversification of indole skeletons through nitrogen atom insertion: B. Morandi et al, Science 2022, 377, 1104-1109
- Aromatic nitrogen scanning by ipso-selective nitrene internalization: M. Levin et al, Science 2023, 381, 1474
- Ring enlargement in the photolysis of phenyl azide: W. Doering et al, Tetrahedron 1966, 22, 81–93
- Conversion of aryl azides to aminopyridines: N. Burns et al, J. Am. Chem. Soc. 2022, 144, 17797–17802
- An efficient synthesis of 3-pyridyl-N-oxide steroids: inhibitors of 5-a-reductase: C. Haffner Tet. Lett.1994, 35, 1349-1352
- Skeletal editing of pyrimidienes to pyrazoles by formal carbon deletion: R. Sarpong et al, J. Am. Chem. Soc. 2022, 144, 22309-22315
- Sodium azide- sonic boom boy: J. Studley blog article March 2023, https://www.scientificupdate.com/process-chemistry-articles/sodium-azide-sonic-boom-boy/
- Synthesis and explosion hazards of 4-azido-l-phenylalanine: M. Richardson et al, J. Org. Chem. 2018, 83, 4525-4536
- https://www.scientificupdate.com/training_courses/safety-selectivity-in-the-scale-up-of-chemical-reactions/








