Whilst I was working towards my PhD in the early 90’s I remember reading ‘The Hot Zone’ by Richard Preston.1 Although non-fiction it read very much like a Michael Critchton2 thriller- describing the emergence and spread of viral hemorrhagic fevers and filoviruses such as Ebola and Marburg. Viruses such as Ebola are somewhat self-limiting in that the infection develops rapidly and has a very high mortality rate.3 Rapid containment and isolation of infected individuals limits the spread of the disease, and the virus burns itself out. Preston hints at a highly probable future scenario in which a virulent airborne virus becomes a global pandemic, with exponential infection rates and overwhelmed, impotent health care systems. Present day- enter COVID-19.4 Although the mortality rates are much lower than Ebola, the rapid global spread and battle to contain coronavirus feels very much like real life imitating conjecture.
COVID-19 emerged, and continues to spread, very rapidly. Alongside the search for an effective vaccine a number of approved and experimental anti-viral small molecule drugs are being tested for activity against the virus.5 Included in this assessment is Favipiravir (Avigan, Favilavir, Figure 1), a compound developed by the Japanese company Toyama (part of Fujifilm) ostensibly to treat influenza and approved in 2014 for ‘treating viral strains unresponsive to current antivirals’.6 Following accelerated approval for development as an experimental treatment for COVID-19 infections, subsequent testing in 340 individuals in Wuhan and Shenzhen has delivered promising results.7 Those who were given the drug in Shenzhen tested negative for the virus after a median of four days post infection, whilst it took a median of 11 days for those without drug. Improvements in lung condition in around 91% of the patients who were given the drug were confirmed by X-ray- against 62% for baseline untreated patients.8 Zhejiang Hisun Pharmaceuticals, who licence the drug in China, are significantly ramping up production.
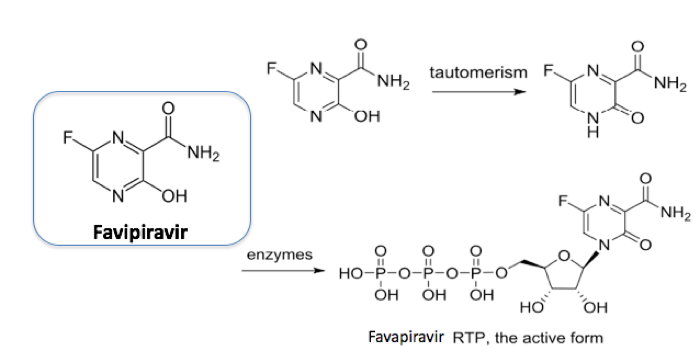
Favipiravir functions (in influenza treatment) by selectively inhibiting viral RNA-dependent RNA polymerase. An alternative postulated mechanistic pathway is through lethal RNA-transversion mutation resulting in a nonviable viral phenotype. The molecule is a prodrug and is metabolized via intracellular enzymes (hypoxanthine guanine phosphoribosyltransferase (HGPRT) is believed to play a key role in this activation process) to generate ribofuranosyl-5′-triphosphate (favipiravir-RTP) (Figure 1).9 The molecule is active in a number of pathogenic RNA viral infections (including a potential treatment for Ebola).
It’s a relatively simple molecule in terms of structure, so the synthetic battle with complexity inherent in some modern antiviral compounds is somewhat muted. However the required volume of drug is now significantly higher than originally anticipated placing additional demands on a robust, scalable synthetic route of manufacture. Several routes to Favapiravir have been described. Most are buried in patents written in Japanese, however the state of the art was nicely summarized by Xie et al last year, together with some of his teams refinements to the synthetic approach.10 I’m sure he had little idea how important this work might be when he submitted his paper back in June 2018.
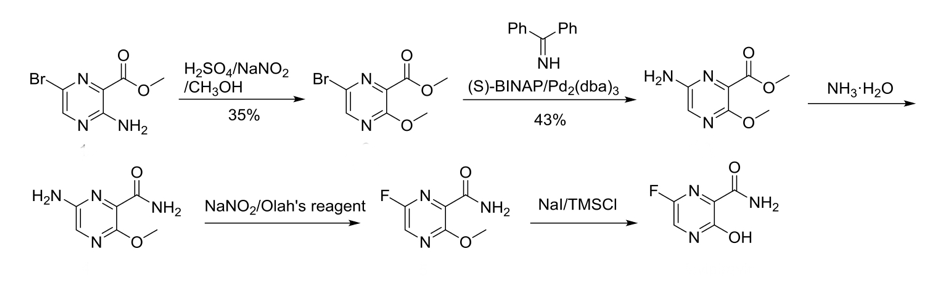
The original synthetic route disclosed in the Favipiravir composition of matter patent is shown below (Scheme 1).11 Diazotization and alcoholysis of the aminocarboxylate (in c. sulfuric acid) gave a low yield of the corresponding methoxy derivative (35%). Palladium-catalyzed imine coupling and hydrolysis (43%) followed by aminolysis of the methyl ester gave the 6-amino fluoro precursor. HF diazotization using Olah’s reagent (70% HF/pyridine complex) followed by deprotection of the methoxy gave Favipiravir. Yields for the final steps are a little difficult to extract from the patent.
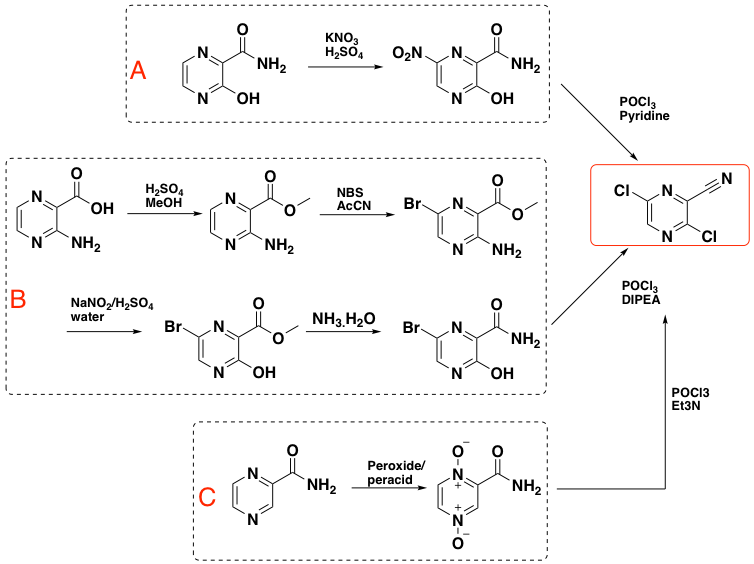
Three subsequent routes rely on the same synthetic late-stage intermediate- 3,6-dichloropyrazine-2-carbonitrile (Scheme 2).12 The first (A) is a two-step sequence involving nitration of the hydroxy-intermediate followed by chlorination/amide dehydration. Though attractive, the nitration yield is very low (18%) and the starting material expensive. A cheaper route (B) starting from 3-aminopyrazine-2-carboxylic acid furnished the cyano intermediate in 37% yield (Scheme 2). The final route (C), from pyrazine-2-carboxyamide, involves oxidation of the pyrazine nitrogen atoms with a peracid or peroxide and chlorination/dehydration. Incidentally 3,6-dichloropyrazine-2-carbonitrile is an allergen, which most likely introduces additional challenges in its manufacture.10
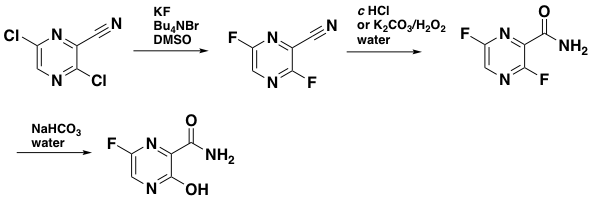
Processing of the dichloro intermediate then involves a HALEX reaction with KF in the presence of a phase transfer catalyst in DMSO at 55°C, hydrolysis of the nitrile group and selective hydrolysis of the labile C-3 fluorine, giving Favipiravir in around 65% over this telescoped 3-step sequence (Scheme 3)- presumably the GMP sequence for manufacture.12
The recent publication by Xie that I refer to above focuses on synthetic routes to the dichloro (or bromochloro-) nitrile intermediate avoiding the use of phosphorus oxychloride vide infra.10 This phosphorus reagent, and in particular work-up of reactions using it in excess, can cause issues for scale-up. Very exothermic quenching, often with delayed onset, has caught out many unsuspecting researchers. The starting material in this case was 2-aminopyrazine which is cheap and readily available. The 4-step sequence to the dichloro-nitrile was achieved in 48% overall yield over 4 steps (Scheme 4). The material was converted to Favipiravir using the HALEX procedure outlined above.
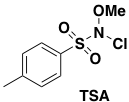
Initial chlorination of 2-aminopyrazine using NCS (in chloroform) proved unsatisfactory due to low yields and formation of impurities. Acetonitrile proved to be a better solvent, however the reaction yield peaked at around 50%. Addition of an excess of NCS resulted in over-chlorination and formation of polymers. The team solved the problem by utilising a reagent developed by Pu et al– N-chloro-N-methyoxy-p-toluenesulfonamide (TSA, Figure 2).13
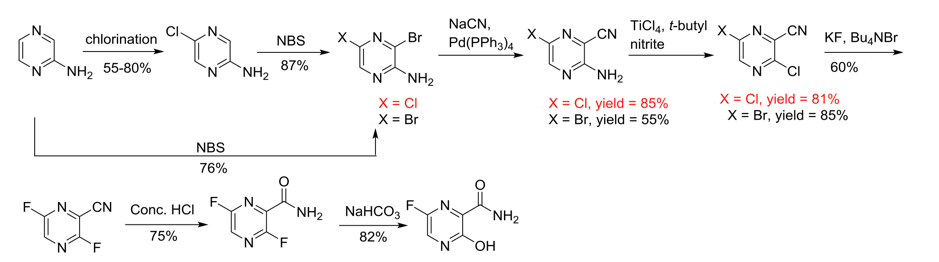
A slight excess of the sulfonamide reagent gave an 80% yield of the required 5-chloro intermediate. Bromination at C-3 with NBS (in DCM) was high yielding (87%). Palladium-mediated coupling of the bromide with sodium cyanide (1.2 eqv, 0.01 eqv Pd(PPh3)4, 0.5 eqv CuI, 10hrs, 120°C) gave the nitrile in 85% yield. Synthesis of the required intermediate was completed by a Sandmeyer reaction using t-butyl nitrite in the presence of titanium tetrachloride (81% yield). Scheme 4 also highlights the attempt to utilize the dibromopyrazine derivative in the cyanide coupling reaction (obtainable from 2-aminopyrazine and NBS in 76% yield). Significant quantities of the unwanted regio-isomer were obtained under the optimized coupling conditions- the required isomer yield being 55%.
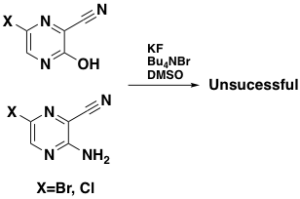
I mentioned earlier that 3,6-dichloropyrazine-2-carbonitrile is allergenic- a couple of attempts to install the 6-Fluoro substituent on electron rich precursors were, not surprisingly, unsuccessful (Scheme 5).
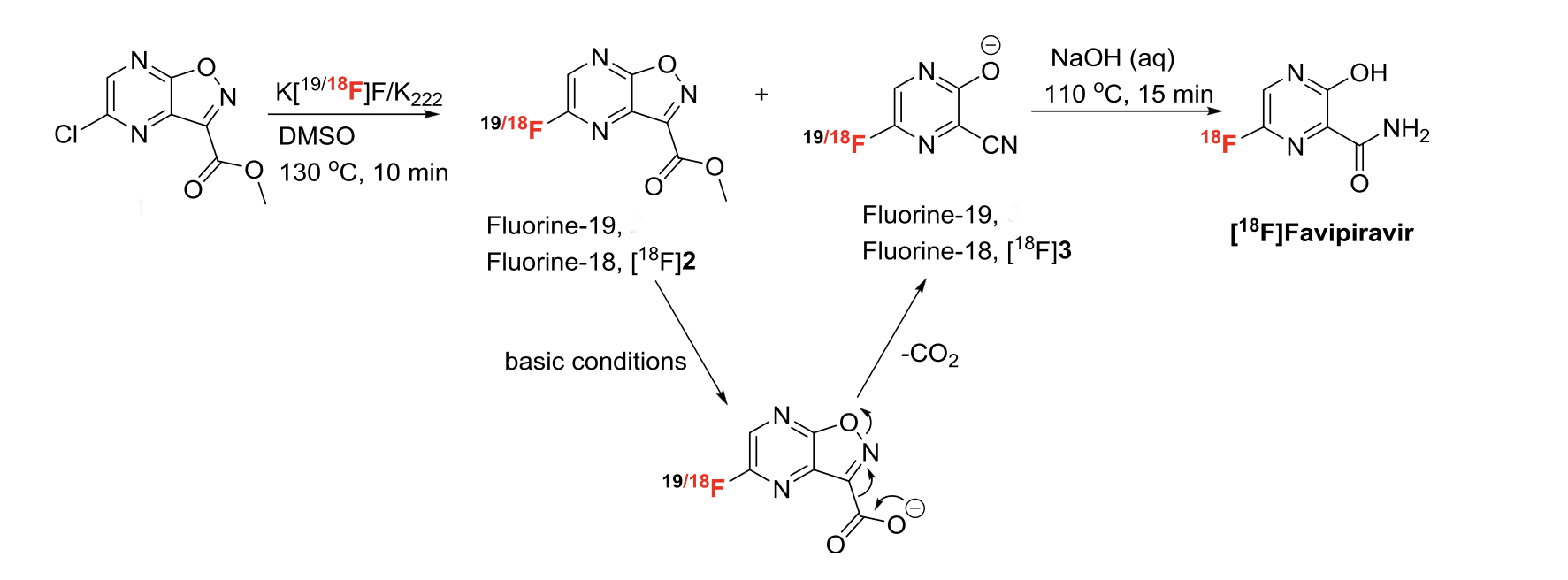
A synthesis of [F18]-Favipiravir has also been published as a one-pot, two-step process from methyl-5-chloroisoxazolo[4,5-b]pyrazine-3-carboxylate (Scheme 6 ).14Here rapid late stage incorporation of 18F is essential due to its short half-life (110. min). The labelled compound was used to characterize the biodistribution of the molecule dynamically using PET.
The continuing battle against emerging pandemic viruses, particularly COVID-19, involves us all. It remains to be seen if Favipiravir can improve the eventual global outcome of this disease. As synthetic chemists we continue to have a role to play in shaping medical history.
“And sometimes he thought of a favourite saying a remark by Louis Pasteur, “chance favours the prepared mind”
― Richard Preston, The Hot Zone, 1994
I sincerely hope you and your loved ones stay safe in these uncertain times.
References:
- “The Hot zone”, 1994, Richard Preston. Preston also wrote similar book on the rise and fall of Smallpox: “The Demon in the Freezer” 2002, Richard Preston.
- ‘The Andromeda Strain’, 1969, Michael Crichton.
- Therapeutic strategies to target the Ebola virus life cycle: T. Hoenen et al, Rev. Microbiol. 2019, 17, 593–606; Perspectives towards antiviral drug discovery against Ebola virus: A. Ali et al, J. Med. Virol. 2019, 91, 2029‐ 2048.
- For an excellent summary of what we know about COVID-19 see coronavirus science: what we know (and don’t know) about the virus: Roger Highfield: https://www.sciencemuseumgroup.org.uk/coronavirus-science-what-we-know-and-dont-know-about-the-virus/ ;The Proximal origin of SARS-CoV-2: K. Andersen et al, Nat. Med.2020 doi: 10.1038/s41591-020-0820-9
- Research and development on therapeutic agents and vaccines for COVID-19 and related human coronavirus diseases: C. Liu et al, ACS Cent. Sci. 2020, 6, 315-331.
- Influenza virus polymerase inhibitors in clinical development: F. Hayden et al, Curr. Opin. Infect. Dis. 2019, 32, 176-186.
- Japanese flu drug ‘clearly effective’ in treating coronavirus says China: Justin MuCurry, The Guardian, 18th March 2020
- China: Avigan effective in in tackling coronavirus: NHK World-Japan March 17th, 2020: https://www3.nhk.or.jp/nhkworld/en/news/20200317_48/
- Mechanism of action of T-705 ribosyl triphosphate against influenza virus RNA polymerase: H. Sangawa et al, doi: 10.1128/AAC.00649-13
- The complete synthesis of Favipiravir from 2-aminopyrazine: Y. Xie et al, Chem. Pap. 2019, 73, 1043-1051.
- Nitrogenous heterocyclic carboxamide derivatives or salts thereof and antiviral agents containing both: WO0010569 (issued March 2000).
- Method for producing dichloropyrazine derivative: WO201008711 (issued August 2012); Synthetic method of favipiravir: CN107226794A (Issued October 2017).
- N-chloro-N-methyoxy-p-toluenesulfonamide: a chlorinating reagent: X. Pu et al, J. Org. Chem. 2016, 36, 5937-5940.
- [18F] Favipiravir and Biodistribution in C3H/HeN mice as assessed by positron emission tomography. Sci. Rep. 2019,9, 1785.
- PYRAZINO[2,3-D]ISOOXAZOLE DERIVATIVE: EP2639235.








