The Lindlar reduction of alkynes to alkenes is well known but does not always work as well as we would like, so any new method for carrying out this potentially useful reduction is always of interest.
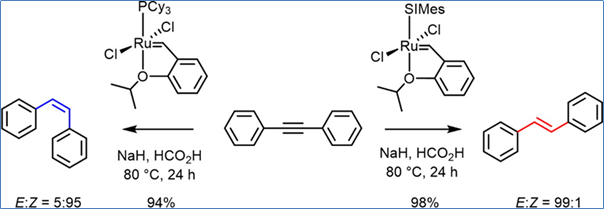
Grela and a co-worker found that Hoveyda-Grubbs HG metathesis catalysts in the presence of NaH (20 mol %) and formic acid will hydrogenate olefins. This particular paper describes the extension of this reaction to alkynes. Interestingly using the generation 1 HG catalyst (with a tricyclohexylphosphine ligand) gives primarily the cis-alkene, whereas if the generation 2 HC metathesis catalyst (with an NHC ligand) is used the major product is the trans-alkene (see Scheme 1). Selectivities are typically 95:5 / 5:95 or better.
Scheme 1: Transfer semi-hydrogenation of alkynes
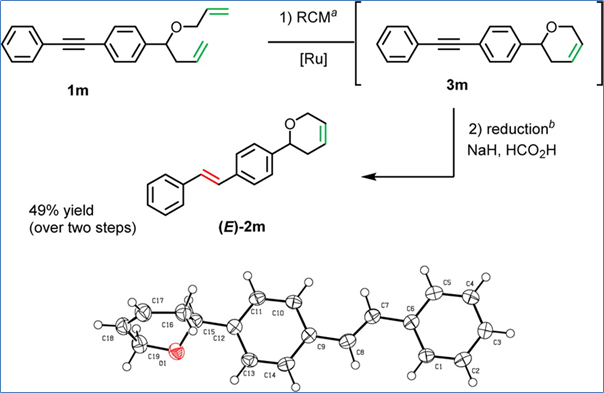
They also extended this reduction to a one pot process where a ring closing metathesis step is carried out followed by semi-hydrogenation of an alkyne as shown in Scheme 2 below.
Scheme 2: One pot ring closing metathesis – alkyne semi-reduction
R. Kusy and K. Grela,* Org. Lett., 2016, 18, 6196-6199.








