A recent paper from a Dutch team brings together two items that have fascinated me since graduate school in a way that comes in the category of “I can’t believe nobody thought of that before!” (Chem. Commun., 2023, 59, 3838)
- The ability to upgrade the enantiomeric excess of a solid by recrystallization
- The use of Soxhlet apparatus
The different crystal behavior of racemic mixtures and enantioenriched mixtures became clear to me during a natural product synthesis that I completed in my graduate studies. I first prepared the target as a racemate to figure out the best yielding reaction conditions for all the steps, then I completed the chiral synthesis on the more valuable enantiomerically enriched material. Interestingly, many of the intermediates that had been oils in the racemic synthesis became crystalline solids in the chiral synthesis, and vice versa! In the short term this was frustrating because I had spent quite a bit of time developing the recrystallization methods during the racemic synthesis so that I could rapidly get through the chiral synthesis and write up a paper, but in the long term it kindled my interest in all the different ways crystallization can be used to improve the purity of materials
In about 90% of cases 50:50 mixture of enantiomers will crystalize as a racemic compound where each crystal contains a 50:50 mixture of each enantiomer. However, in about 10% of cases a conglomerate is formed; in which each crystal contains only a single enantiomer, but there are equal numbers of each type of crystal. Louis Pasteur was the first to recognize this when carefully observing that there were two different types of crystal that formed from sodium-ammonium tartrate, and then manually separated them to start the field of chiral chemistry.
If the two enantiomers in a conglomerate system can be interconverted in solution and the solid mixture is not 50:50, then over time the solids present in the system will become enriched in the enantiomer that was initially present in excess. This chiral amplification has been proposed as an explanation for why most biological building blocks are found as single enantiomers and has also been exploited in the fine chemical and pharmaceutical industries to upgrade the enantiopurity of isolated materials.
In a previous paper the authors showed that the best way to upgrade the enantiomeric excess of the solid with temperature cycling was to slowly grow the crystals with deep cooling then rapidly heat the system which would preferentially redissolve the smaller crystals (i.e., the ones consisting of the remaining minor enantiomer). Unfortunately, a cycle of slow cooling followed by rapid heating is difficult to achieve without creating hot spots and possibly overshooting the desired temperature. So instead, they conceived of a process where the crystals would be grown by slow removal of solvent, then hot solvent would rapidly be re-added, and the cycle repeated.
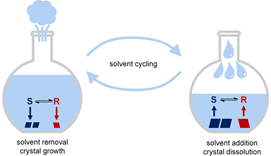
One could envision several “modern” ways of achieving this through an expensive computer-controlled system, but with great insight the authors realized that a piece of glassware invented in 1879 by Franz von Soxhlet would do just as well!
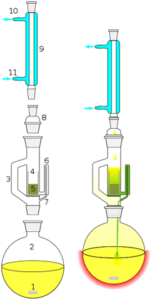
Traditionally, a Soxhlet apparatus is used to extract soluble materials from a solid: during my graduate work that was a triol that was prepared by a global reduction of a keto diester using lithium aluminum hydride, but which stuck tenaciously to the aluminum salts after the reaction was quenched. After unsuccessfully trying to extract the triol from the solid using a range of solvents at room temperature someone introduced me to a Soxhlet extractor and that afternoon I was able to obtain more triol than I had in the previous month. The diagram below shows how a Soxhlet extractor is typically used.
The extraction solvent (1) is placed in a flask (2) and heated to reflux. Solvent vapor rises through side arm (3) and is condensed by the reflux condenser (9) and hot liquid then falls into the Soxhlet reservoir which contains the solid sample (5) in a thimble (4) made of a porous material. This means that the solid is extracted by hot solvent, but the clever part of the Soxhlet apparatus is the side arm (6). As the solvent level rises it fills the side arm until it reaches the top of it (>5 min), at which point the siphon tube (7) sucks all the liquid rapidly (<10 sec) out of the Soxhlet reservoir and back into the heated flask. Then the cycle starts again, which enables repeated unattended hot extraction of the solid, rather than the laborious (and more hazardous) series of hot extractions that I had been trying for weeks. There is something hypnotic and thrilling about watching the side arm fill with solvent then all the contents suddenly and rapidly drain back into the reactor.
To see this in action watch the video in the link below (it’s much clearer than my wordy description) https://upload.wikimedia.org/wikipedia/commons/3/32/Soxhlet_siphoning.webm
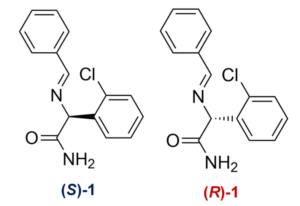
The conglomerate system studied in the paper is an imine precursor (1) to clopidogrel which can be readily racemized in solution by adding an organic base, such as DBU in this case.
Choice of solvent is critical. Usually, this molecule is deracemized in MeCN but the high boiling point of the solvent has three drawbacks
- Decomposition of material in solution
- Low yield because a significant amount of material remains in solution
- Fast crystal growth leading to low chiral enrichment due to a steep increase in supersaturation at high temperatures
In this study the authors used empirical solubility data in different binary mixture ratios and simulation methods to select a binary mixture of diethyl ether and acetone in order to maximize the amount of solid that grows during evaporation but then is subsequently redissolved by re-addition of the solvent. The deracemization was performed on 800 mg of a 55:45 R:S enantiomer mixture of Compound 1 in 25 mL of a 90:10 mixture of diethyl ether:acetone heated to 50 °C with DBU as catalyst. After 18 h, the solid was obtained in 90% yield at 99.5:0.5 R:S!
The paper concludes with kinetic studies exploring the rate of deracemization with starting enantiomer ratios, and also shows that the process has no substantial kinetic bias towards either of the two enantiomers. Control experiments ruled out metastable crystal transformations or attrition as the mechanism, and showed that without the Soxhlet apparatus there was virtually no deracemization. Comparison to other well-established methods of deracemization shows that this method is faster, despite only brief optimization studies. The authors conclude by stating that they foresee several ways to design new deracemization systems that were previously ineffective or impossible, and that solvent cycling processes should be readily scaled-up in conventional equipment. A plant-scale Soxhlet extractor sounds to me like a really fun piece of equipment to see in action!








