The introduction of bromine into intermediates is a key transformation in the synthesis of pharmaceuticals and other high value materials, due to the reactivity and selectivity it offers for further transformation, over for example the generally cheaper, more common but less reactive chlorinated analogues. Bromine is commonly introduced by one of three reagents: bromine itself (Br2), HBr, or N-bromo-succinimide (NBS), all with or without mild oxidants, although other methods are available (see the ACS Green Chemistry Institute Pharma Roundtable on Brominations for an overview – https://reagents.acsgcipr.org/reagent-guides/bromination/list-of-reagents).
Bromine itself is a difficult reagent to handle in both lab and plant, being a dense corrosive fuming liquid which is incompatible with many non-glass materials, whilst HBr in acetic acid is often incompatible with other functionalities. Solid, crystalline NBS may be preferred as relatively mild, although the purity, colour and assay of NBS often needs to be checked before use. On scale, solids are usually charged first, although even this can be problematic with NBS – a colleague once weighed NBS onto the substrate and observed a solid-state reaction between the two, liberating brown fumes, quickly transferred to the fume cupboard! Generally, it is undesirable and difficult anyway to charge large quantities of solids to partially filled reactors when trying to mimic continuous addition.
To achieve dose-controlled addition, often required for both safety and selectivity, solids like NBS are dissolved in solubilising polar aprotic solvents like DMF. Yang et al. have recently reported on the hazards of using DMF with various reagents, particularly oxidants and Br2 (in summary, DON’T!) (Org. Process Res. Dev. 2020, 24, 1586−1601; https://doi.org/10.1021/acs.oprd.0c00330) – it is more reagent than solvent. (We generally recommend on our courses avoiding DMF for sustainability and toxicity reasons, as much as due to its reactivity). Shimizu et al. have previously reported on the incompatibilities between NBS and various solvents (Org. Process Res. Dev. 2014, 18, 354−358; https://doi.org/10.1021/op400360k). However, a hazard group at Hovione felt there was still some uncertainty over the NBS/DMF combination, perhaps under pressure of project delivery, and so conducted more extensive hazard testing on a 22wt % solution of NBS in DMF to support scaling up a Wohl−Ziegler reaction (allylic and benzylic bromination) (Gonçalves et al., Org. Process Res. Dev. 2023, 27, 1975−1983; https://doi.org/10.1021/acs.oprd.3c00104) (The use of neat Br2 was unselective in their case leading to dibromination, which is not uncommon).
Starting with an RC1 test, the group found that when adding NBS to DMF, upon reaching 80 °C, an isothermal decomposition occurred releasing 149 kJ/kg and 4.2 L/kg of off-gases and a maximum achievable temperature of 161 °C under assumed adiabatic conditions typical of large-scale reactors. This is above the bp of DMF (153 °C). However, a complex light-promoted, radical-mediated decomposition of NBS was thought likely to be occurring, potentially also decomposing DMF. Furthermore, the data also suggested auto-catalytic decomposition. On this basis, further testing was initiated, especially to determine the TD24 (temperature of safe operation allowing 24 hrs to thermal runaway).
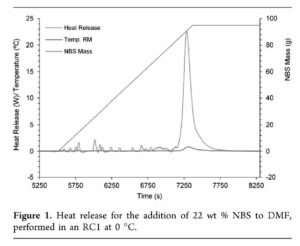
Initial DSC data suggested a TD24 of -1 °C. DSC scans were also performed at several concentrations of NBS in DMF which showed a concentration dependence, with (unsurprisingly) higher energies and slightly earlier onset temperatures for higher concentrations. These tests were run in standard gold DSC crucibles, assumed to be inert to the materials tested. However, much standard plant is made of stainless steel so stainless steel crucibles were also trialed, again revealing higher energies and lower onset temperatures compared to the gold crucibles, although re-analysis suggested decomposition had been autocatalytic in the gold pans. A Carius tube test was then also run to demonstrate compatibility with glass. Neither DSC nor Carius tubes are especially sensitive of techniques, but even so, both did provide valuable data and insights into the stability of NBS/DMF solutions.
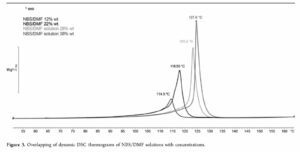
Additional testing using ARC (accelerating rate calorimeter) and RSD (Rapid Screening Device, also ARC based) techniques in Hastelloy bombs provided further data, including gas generation, which the DSC and Carius tube techniques don’t easily provide. From all this data, a kinetic model was constructed with summary results of an adiabatic temperature rise (DTad) of 90 K and a TD24 of 32 °C for 22 wt% solutions of NBS in DMF.

What I particularly liked about this study was the exemplary manner in which these hazard tests were conducted, combining the various experimental techniques (DSC, Carius tube, ARC, RSD and RC1) with their different strengths and limitations, to determine the key parameters of DTad and TD24, all within the framework of the Stoessel classifications (Stoessel, Thermal Safety of Chemical Processes: Risk Assessment and Process Design, Wiley-VCH, 2020). It’s also worth noting how concentration of reagent solution and Materials of Construction (MoC) affected the hazard results – factors of which to be aware.
Actually, I think I would go further than these authors given these results, and suggest that NBS/DMF solutions should be avoided. The somewhat similar 1,3-dibromo-5,5-dimethylhydantoin (DBDMH), widely used in water treatment and paper bleaching, might be a better option. Perhaps this or another group else could serve the chemical community by determining hazard data on the stability of DBDMH in various solvents first.
This case study and others featuring useful practical examples feature in many of our training courses: https://www.scientificupdate.com/training/courses/








