A patent issued this month cites the use of catalytic tetrabutylammonium bromide (TBAB) in the ring opening esterification of glycidyl ethers using acrylic acid and methacrylic acid (see Example 7). In PTC esterifications, we typically expect the presence of a base to form the carboxylate salt from the acid then the quat acrylate would be more reactive as a looser ion pair than say sodium acrylate, even assuming that all components are fully soluble in acetonitrile.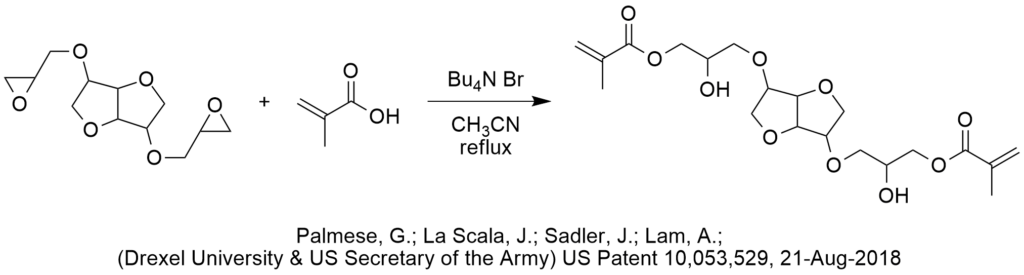 However, the procedure in this patent does not report the use of any base. It is possible that the acid protonates the epoxide which makes it susceptible to attack by the bromide to form the bromohydrin which may be more reactive than the closed epoxide. We find it surprising that the inventors did not titrate the acrylic acid/methacrylic acid with a weak base that is also a desiccant which would enable the loose quat-acrylate ion pair to be more effective. If any of our readers have a better explanation of how the quat bromide is catalyzing this reaction, please contact Marc Halpern with your input. By the way, sometimes quat bromides are known to catalyze reactions of epoxides by having the bromide attack an epoxide to form a bromoalkoxide that reacts further. However, in this case it is not clear that this would help form the desired product. |








