If you had suggested 10 years ago that it would be a good idea to try and develop some of the reactions that you can now carry out using photoredox catalysis, you would probably have been labelled as deluded, if you were not locked away for your own safety. But photoredox catalysis has come a long way and now enables us to carry out transformations that often look impossible, and usually under pretty mild conditions. Join our webinar on Friday 27th April to learn more about the following reactions and much more. Click HERE for more information on our FREE webinar
Oxidation alpha to nitrogen seems sensible, but oxidation at the beta position? Well that does work using sodium decatungstate as the photo-catalyst and hydrogen peroxide as the terminal oxidant at room temperature. Scheme 1. D.M. Schultz et al (Merck), Angew. Chem. Int. ed., 2017, 56, 15274.
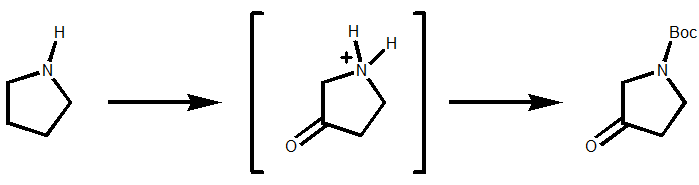
Scheme 1
Direct fluorination of unactivated C-H bonds also looks an unlikely transformation but is feasible with NFSI as the fluorinating agent and tetrabutylammonium decatungstate as the photocatalyst. Scheme 2. S.D. Halperin et al, Angew. Chem. Int. ed., 2014, 53, 4690
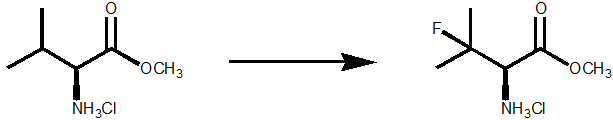
Scheme 2
Finally the tricycle formation is, in principle, possible under standard radical conditions using AIBN, but the starting material decomposes at the temperatures required to generate radicals. Using photoredox catalysis with Iridium catalyst gives the desired product in 50% yield. Scheme 3. P.P. Zhang et al, J. Am. Chem. Soc., 2017, 139, 13989
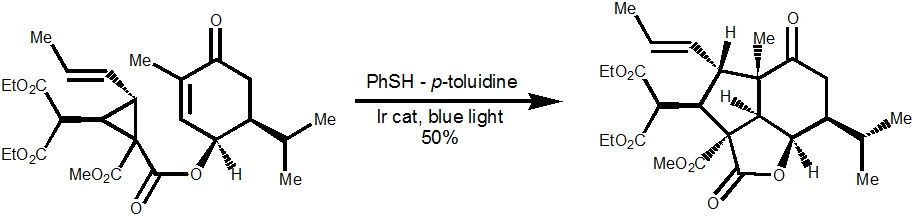
Scheme 3








