The generation of lithium-alkoxide based aryl and heteroaryl Grignard reagents by Mg-Br/Cl exchange in toluene- increased reactivity and wide applicability.
Organomagnesium-based organometallics are used extensively in organic chemistry.[1] Historically the reagents were prepared by direct insertion of magnesium metal into organic halides. With demand for more industrially applicable methods, halogen-magnesium exchange using alkyl magnesium halides or turbo-Grignard (iPrMgCl.LiCl) have become widely adopted for aryl or heteroaryl iodide or bromide substrates.[2] The choice of solvent for these reactions is generally restricted to ether-based solvents, which co-ordinate and stabilize the organometallic.
Many Grignard reagents are commercially available as solutions in THF or diethylether. These water-miscible solvents can cause issues in down-stream processing during work-up and extraction. The ability to generate Grignard reagents in non-polar, greener, hydrocarbon solvents such as toluene would address this issue to some extent and in addition give potentially different reactivity in the absence of a magnesium-co-ordinating ether.
The Knochel team, building on their seminal work in developing the turbo-Grignard reagent, have published details of two new halogen-magnesium exchange reagents (sBuMgOR.LiOR and sBu2Mg.2LiOR) that undergo rapid Mg/Br exchange and, for the first time, Mg/Cl exchange with electron rich aryl chlorides (Angew. Chem. Int. Ed. 2018, 57, 6701). Both reagents were prepared from nBu2Mg by generation of the alkoxymagenesium intermediate ((RO)2Mg) using 2-ethylhexanol and addition of sBuLi (1 or 2 equivalents respectively), removing solvent and re-dissolving in toluene to give a 1.0-1.5M solution of the monomeric complex and a 0.6-0.85M solution of the dimeric species.
The paper describes a head to head comparison of the magnesium exchange reaction with 4-bromoanisole and iPrMgCl.LiCl (THF), sBu2Mg.2LiCl (THF) and sBuMgOR.LiOR (in both toluene and THF). In general, the exchange is 30 times faster than sBu2Mg.2LiCl and 110 times faster than iPrMgCl.LiCl (turbo Grignard) with the new alkoxide reagent. Addition of TMEDA or PMDTA (pentamethylenediethylenetriamine) gave an improved conversion, and a clear reactivity advantage in running the reaction in toluene was demonstrated. The procedure was also applied to preparation of heteroaryl magnesium alkoxides.
The proposed structure of the magnesium reagent is shown(figure). A range of reactions were demonstrated with the magnesium species including trapping with various electrophiles, transition metal mediated couplings and interestingly ring opening of epoxides and aziridines. The later often require Lewis acids and forcing conditions, however the alkoxide performs under mild conditions (25°C, 4-10hrs).
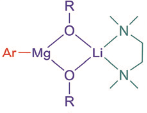
Proposed structure of exchange reagent (R=2-ethylhexyl), Mg/Br and Mg/Cl
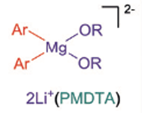
Chloro-magnesium exchange, something of a holy grail for organomagnesium chemistry was also explored. The dimeric complex (sBu2Mg.2LiOR) showed reactivity where the other reagents failed and afforded the bis(magnesium) species shown (figure). This complex showed the expected reactivity in several trial reactions with electrophilic reagents. With the wider commercial availability of chloro-aryl substrates this result bodes well for future development in this area.
[1] Grignard reagents New Developments 2000 Wiley-VCH Ed. Herman G. Richey; Organometallics 2009, 28(6), 1598-1606
[2] Progress and development in turbo-Grignard reagent i-PrMgCl.LiCl: a ten year journey Chem. Commun. 2015, 51, 6884-6900
For more information on an organometallic chemistry training course run by Scientific Update on stoichiometric organometallics such as Grignard reagents and catalytic organometallics based mainly on palladium and other platinum group metals check out our website.








