Several weeks ago I did my annual analysis of the synthetic routes used to prepare small molecule drugs approved by the FDA in the preceding year, obviously in this case 2022.1 I hope those of you who have seen the presentation found it as interesting to watch as I found to put it together. One of the questions I was asked by an attendee related to an unusual CBz deprotection method used in the synthesis of the KRAS -G12C inhibitor Adagrasib , developed and marketed by Mirati Therapeutics (Figure 1).2 The method is a little unusual. It involves nucleophile attack at the CBz- benzylic carbon with a thiol, and, as it turns out, the process chemistry team who developed the methodology published an Org. Lett paper in 2022 describing the generality and scope of the process together, with a mechanistic hypothesis.3
Used to remove the CBz group in the manufacture of Adagrasib, both the nitrile and the aryl chloro- are unaffected (to any great extent) by the thiol reaction (Figure 1). Since the reaction is in the cGMP sequence used to manufacture the API it must be chemically robust and well investigated.2
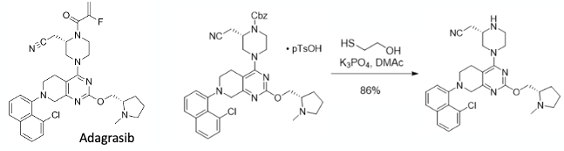
Before I go into more detail some background and context. The general go-to method for cleavage of a CBz group is heterogeneous hydrogenation, typically using Pd/C. In the majority of cases this work extremely well and is used routinely at scale.4 The problem with this approach is that if the substrate contains a reactive functional group, or, in this case an aryl halide (particularly bromides or iodides), competing reduction or dehalogenation can occur generating impurities that on occasion can be challenging to purge. There are obviously alternative methods available. In the paper they highlight a Lewis acid- TMS-iodide- as a possible alternative, however the by-product (benzyl iodide) is a powerful alkylating agent that could react with reactive functional groups.5 From a safety perspective its highly likely genotoxic so controlling the level of residual material to low ppm levels in the final API introduces challenges. If the reaction is used late in the synthesis (as is the case with Adagrasib) this compounds the issue and becomes a significant regulatory concern.
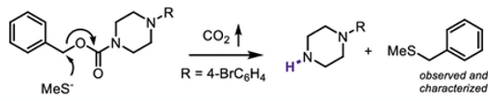
If you need to cleave a Cbz group- and you have a sensitive substrate- you need a toffee hammer rather than a sledgehammer so to speak. The Mirati team came up with a good solution to the problem. Their hypothesis is a simple one. SN2 attack at the CBz benzylic carbon (with a nucleophilic thiol) should generate an amine carbonate. In situ decarboxylation furnishes the required amine and the benzylated thiol (Figure 2). Presumably the latter is relatively volatile so can be removed by distillation. To be honest it’s one of those reactions that in retrospect seems obvious. So much so that one would assume someone else should have tried it before. Evidently not (unless they and the referees missed it). The described method is simple but potentially very effective.
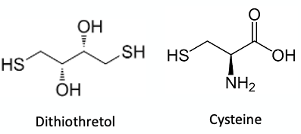
There is fair amount of literature precedent for thiol-mediated dealkylation, particularly of aryl ethers. A variety of thiolates have been used, including protected forms of the amino acid cysteine and dithiothretol (Figure 3).6
The Mirati team confirmed their hypothesis using the simplet thiol they could find-Sodium methanethiolate- with a model substrate- benzyl 4-(4-bromophenyl)piperazine-1-carboxylate (75°C,DMF, 4hrs) .The reaction was clean-the only material present other than the free amine being the BnS-Me thioether.
Proof of concept- tick. The elephant in the room here is the smell of methane thiol. I’ve worked in labs where this material has been generated and resulted in evacuation of the building, despite diligent containment efforts on the part of the hapless chemist. One things for sure. If you’ve worked with the stuff you’ll have no trouble getting a seat on the bus!
Now an interesting fact that many may not be aware of is that as you incrementally increase the length of the alky-thiol chain, by the time you reach C-10 the thiol is odourless.7 SAR around olfactory receptors and all that. The problem is that long-chain thiols tend to give emulsions during work-up and are not particularly atom efficient. After some trial and error the Mirati team identified 2-mercaptoethanol as a suitable nucleophile. Apparently this is only “minimally odiferous”. Wikipedia concurs- the smell is described as unpleasant but not as objectionable as related thiols. The boiling point is relatively low (157°C) and the material is available at scale. If you’re interested it’s made by ring-opening of ethylene oxide with hydrogen sulphide. I’m sure the team were happy they didn’t need to concern themselves with that.8 They don’t comment on possible oxidation to the disulfide but I’m sure it must happen.9
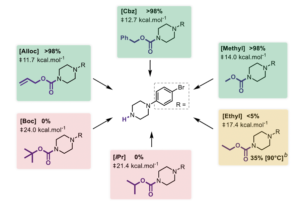
Using optimised conditions (2 eqv 2-mercaptoethanol, 4 eqv. KOAc, DMAC [0.25M], 75°C, 24hrs), a collection of N-alkyl carbamate-protected 1-(4-bromophenyl)piperazine’s were tested for concomitant reactivity (Figure 4). Computational activation energies for the Benzyl, alloc and methyl carbamate were calculated (12.7, 11.7, 14.0 kcal/mol) and consistent are consistent with sp3-hybridised carbon atoms. These substrates worked well. The methyl result is perhaps a little surprising. Having said that thiols are used to cleave aryl methyl ethers, vide supra. Good yields were obtained with these substrates. The difference in reactivity between methyl and ethyl is particularly stark (Figure 4).
Direct attack at the carbonyl was estimated to require much higher activation energy (>50kcal/mol) and thus is an unfavourable reaction pathway.
Simple but effective. Something worth considering if you find yourself on the wrong side of a complex Cbz deprotection.
See you next time.
References:
- Synthesis of the new drugs approved in 2022: J. Studley, archived recording and a pdf of the slides is available on the Scientific update website: https://www.scientificupdate.com/webinar_events/synthetic-approaches-to-the-new-drugs-approved-in-2022/20230126/
- Development of Adagrasib’s commercial manufacturing route: D. Snead et al, Org. Process Res. Dev. 2023, https://doi.org/10.1021/acs.oprd.2c00386
- A nucleophilic deprotection of carbamate mediated by 2‐mercaptoethanol: T. Scattolin et al, Org. Lett.2022, 24, 3736-3740
- W. Green, P. G. M. Wuts, Protective Groups in Organic Synthesis, Wiley-Interscience, New York,1999, 531-537, 736-739
- Iodotrimethylsilane—a versatile synthetic reagent: G. Olah et al, Tetrahedron 1982, 38, 2225-2277
- Recent advances in ether dealkylation: S. Weissman et al, Tetrahedron 2005, 61, 7833-7863; Metal-free C–S coupling of thiols and disulfides: M. Pramanik et al, Org. Biomol. Chem. 2020, 18, 8771-8792
- Odourless substitutes for foul-smelling thiols: syntheses and applications: M. Node et al, Tet. Lett. 2001, 42, 9207-9210
- Production of 2-mercaptoethanol: US3710439A
- Efficient synthesis of disulfides by air oxidation of thiols under sonication: J. L. G. Ruano et al, Green Chem.2008, 10, 706-711








