The ability to handle gaseous reagents presents a number of challenges for synthetic chemists. The problem becomes particularly acute when scaling up biphasic (liquid/gas) or even triphasic (gas/liquid/solid) systems due to limiting mass transfer effects. Pressure vessels or autoclaves are also not always practical and easy to come by. A practical solution to this (if you forgive the pun) is to use a solid “carrier” precursor or surrogate that is much easier to handle and enables us to accurately dose compound to our reaction mixtures. Examples include NCS/NBS form bromine/chlorine, N-F reagents such as selectfluor for fluorine and the DABSO sulfur dioxide reagent developed by the Willis group at Oxford.1 Carbon monoxide is another gaseous material that is very often generated in situ from suitable “trigger molecule” precursors.2 This approach has even been extended to in vivo medical applications and novel therapeutic methods.3
Another reagent that falls into the similar-difficult to handle- category is the C-2 building block ethylene- a gas that has historically been used for functionalization of simple substrates but remains untapped in the synthesis of complex organic substrates. This is somewhat surprising given that annual global production exceeds 100 million tons and the vinyl substituent is a versatile handle for synthetic elaboration. There’s plenty of it around. Its low reactivity dictates the use of high temperatures and pressures so for elaborate substrates it remains on the periphery of complex synthesis. That said metal-driven processes using ethylene have been used successfully, and work through co-ordination / activation type mechanisms.4a Olefin metathesis generates ethylene as a by-product that can inhibit a metal catalyst, however, some interesting work by Jammison and Bio on continuous processes incorporating a gas permeable membrane to mitigate this problem was recently published in the OPRD journal.4b
Interesting aside-ethylene is a plant hormone that causes fruit to ripen. It’s used in the food industry to provide controlled ripening during storage and transport. The banana you ate for lunch was probably ripened on the boat with a squirt of ethylene just before docking!
A possible solution addressing the use of ethylene in synthesis is a recently described vinyl thianthrenium tetrafluoroborate reagent developed by the Ritter team (Figure 1).5 The compound is prepared from ethylene at low (balloon) pressure from commercially available thianthrene-S-oxide via activation with Tf2O (generating an intermediate dication species) and is apparently a non- hygroscopic solid that can be stored at room temperature in air without signs of decomposition for at least one year. The DSC/TGA data (nice to see in an academic paper) shows no worrying thermal activity below 280°C- at which temperature it appears to decompose with evolution of gas. Historically, vinyl bromide has been used as a vinyl source in synthetic chemistry, or other reagents derived from it (-SiMe3, SnBu3, B(OR)2 etc.) The bromide itself a gas and has significant toxicity issues. Acetylene has been used as a vinyl precursor-particularly calcium carbide as a solid acetylene source- with varying levels of success.4c Ritter has published extensively on the synthetic application of thianthrenium salts including trifluoromethylation,6a C-H alkylation,6b, copper-mediated cross coupling reactions,6c late-stage C-H functionalization,6d tritiation of aryl substrates,6e [18F] incorporation,6f electrophilic alkene functionalization,6g and allylic amination of alkenes.6f
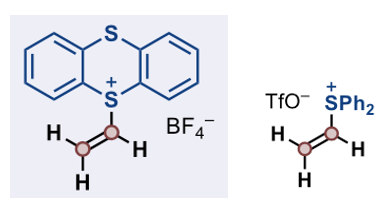
The thianthrenium salt is similar in many ways to the vinyl diphenylsulfonium salts developed by Aggarwal as a 1,2-ethane synthon (Figure 1), however the former is reported to be practically easier to handle and is a solid rather than a hygroscopic oil.7
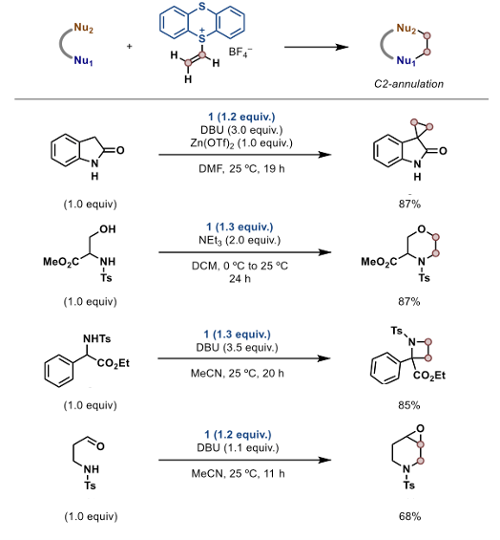
So what can you do with it? Well quite a bit actually. Its use as a 1,2-synthon was demonstrated in a number of hetero- and carbocycles generating cyclopropanes, morpholines, azetidines and epoxides (figure 2).5
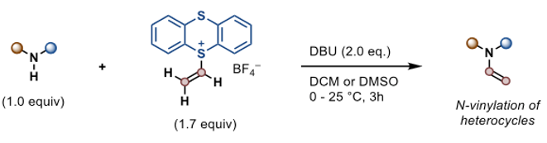
Another very useful transformation that is not easily achieved via other methods is the base-mediated synthesis of N-vinylated nitrogen containing heterocycles, such as indoles, imidazole and pyrazoles. The current method worked well, with good functional group compatibility (aldehydes, nitro groups, esters) being observed hinting at a wide synthetic scope for further elaboration (Figure 3). A light dusting of drugs were also vinylated- seems to be a thing these days to demonstrate you new methodology.
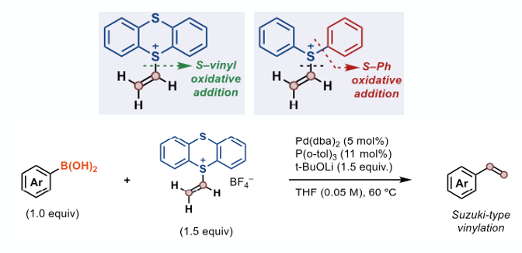
Another particularly interesting application is the synthesis of styrenes. Vinylated arenes have wide utility as synthetic building blocks and industrial feedstocks. Existing metal-based synthetic methods can result in formation of unwanted Heck-type by-products and/or extensive product polymerization. In the case palladium-mediated cross coupling of the diphenylsulfonium salts described by Aggarwal (Figure 1) with aryl boronic acids, competing aryl C−S bond oxidative addition results in formation of a bi-aryl. In the case of the thianthrenium salt, oxidative addition of the of the S-vinyl bond is irreversible whereas oxidative addition of the S-aryl is not (Figure 4). A wide range of aryl boronic acids with different functional groups and substitution patterns were coupled in a Suzuki-type process (Figure 4).
A competition experiment between vinyl thianthrenium –d3 and vinyl bromide using 4-chloroboronic acid as a coupling partner established that the thianthrenium compound reacted significantly faster than vinyl bromide.
Vinyl thianthrenium tetrafluoroborate is a useful reagent to have in the toolbox and addresses the limitations of existing methodology. It’s worth highlighting that a salt counterion can have a big impact on the crystallinity and ease of handling of a reagent or intermediate. The BF4– counterion, I’m sure, helps to facilitate the favourable physical properties of the material.
See you next time.
References:
- Rediscovering the chemistry of sulfur dioxide: new developments in synthesis and catalysis, M. Willis et al, Synthesis 2014, 46, 2701–2710.
- Carbonylations of alkenes with CO surrogates. Wu et al Angew. Chem. Int. Ed. 2014, 53, 6310–6320; Evolution of carbonylation catalysis: no need for carbon monoxide: T. Morimoto et al, Angew. Chem. Int. Ed. 2004, 43, 5580–5588; The development and application of two-chamber reactors and carbon monoxide precursors for safe carbonylation reactions: T. Skrydstrup et al, Acc. Chem. Res. 2016, 49, 594–605.
- Development of triggerable, trackable, and targetable carbon monoxide releasing molecules, L. Berreau et al, Chem. Res. 2020, 53, 2273−2285.
- a) Transition-metal-catalyzed laboratory-scale carbon−carbon bond-forming reactions of ethylene, V. Saini et al, Angew. Chem., Int. Ed. 2013, 52, 11206−11220; b) A scalable membrane pervaporation approach for continuous flow olefin metathesis, T. Jamison & M. Bio et al, Org. Process Res. Dev. 2020, 24, 2298-2303; c) Calcium carbide: a unique reagent for organic synthesis and nanotechnology, G. Werner et al, Asian J. 2016, 11, 965 –976.
- Vinyl thianthrenium tetrafluoroborate: a practical and versatile vinylating reagent made from ethylene, T. Ritter et al, Am. Chem. Soc. 2021, 143, 12992–12998.
- a) Trifluoromethyl thianthrenium triflate: a readily available trifluoromethylating reagent with formal CF3+, CF3, and CF3–reactivity, T. Ritter et al, J. Am. Chem. Soc. 2021, 143, 7623–7628; b) Site-selective C-H alkylation of complex arenes by a two-step aryl thianthrenation-reductive alkylation sequence, T. Ritter et al, J. Am. Chem. Soc. 2021, 143, 7909-7914; Site-selective and versatile aromatic C-H functionalization by thianthrenation, T. Ritter et al Nature 2019, 223-228; c) Enabling the use of alkyl thianthrenium salts in cross‐coupling reactions by copper catalysis, T. Ritter et al Angew. Chem. Int. Ed. 2021,60, 21756 –21760; d) Site-selective late-stage c–h functionalization via thianthrenium salts, T. Ritter et al, Synlett 2022, 33, 339-345; e) Tritiation of aryl thianthrenium salts with a molecular palladium catalyst, T. Ritter et al Nature 2021, 600, 444-449; f) Site-selective late-stage aromatic [18F]fluorination via aryl sulfonium salts, T. Ritter et al Angew. Chem. Int. Ed. 2020, 59, 956-1960; g) Regio‐ and stereoselective thianthrenation of olefins to access versatile alkenyl electrophiles, T. Ritter et al Angew. Chem. Int. Ed. 2020, 59, 5616-5620; h) Allylic amination of alkenes with iminothianthrenes to afford alkyl allylamines; T. Ritter et al, J. Am. Chem. Soc. 2020, 142, 7287-17293
- Sulfonium, ethenyldiphenyl-1,1,1-trifluoromethanesulfonate, V. Aggarwal et al, Encyclopedia of reagents for organic synthesis 2012, 1−6; ibid 2016, 1-5.








