Any new synthetic methodology, as well as advancing the state of the art, should also consider a few other key criteria, one of which is to build complexity from common feedstock materials- ideally using cheap and environmentally friendly reagents and raw materials. Unactivated (or unsubstituted) olefins, used largely by the polymer industry, represent a rich pool of materials primed for oxidative processing to useful intermediates. Despite their ubiquity in nature and in the chemical industry, the preparation of complex small molecules from these commodity materials remains an ongoing synthetic challenge. That said, a number of groups are actively engaged in utilising these materials.
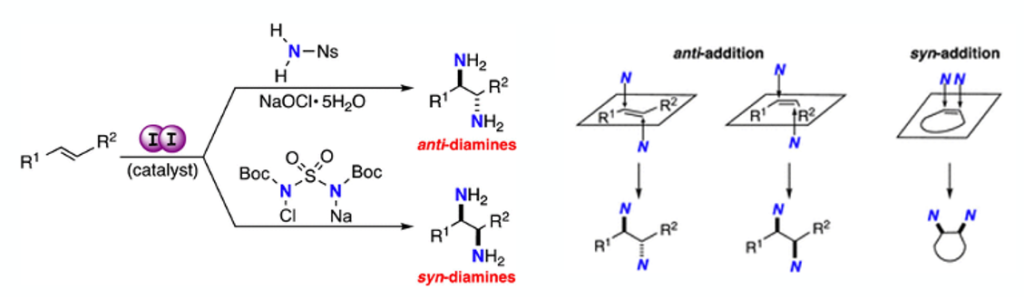
A good example is a recent publication by Minakata et al in which he describes conversion unactivated E/Z olefins to both anti-and Syn- 1,2-diamines respectively (Figure 1) using catalytic iodine in combination with a suitable nitrogen source, chloramine-Ns (nosylamide), and a somewhat underutilized oxidant- solid sodium hypochlorite pentahydrate.1
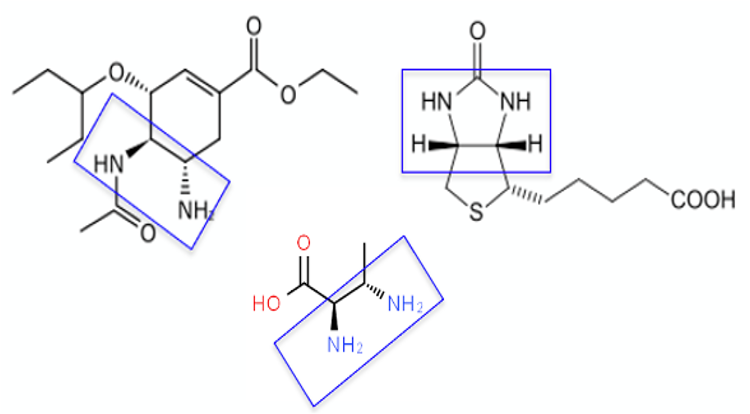
As well as being important ligands for both asymmetric transition-metal catalysed and organocatalytic reactions, the 1,2-diamine architecture features in a number of drugs and natural products, including the neuraminidase inhibitor Oseltamivir, the B7-vitamin biotin and the unnatural amino acid trans-2,3-diaminobutyric acid (Figure 2).2
Just a brief aside: asymmetric dihydroxylation of alkenes- the normal stopping ground of osmium-3 has just been reviewed by Su and Wang in Synthesis from the perspective of osmium-free alternatives. Well worth a look.4
Historically, the nitrogen versions of the alkene dihydroxylation reaction are the Prevost and Woodward reactions. Metal-mediated synthesis of 1,2-diamines under stochiometric conditions was developed in the 1970’s. Ironically, catalytic variations proved difficult due to the effectives of the products as metal binding ligands. Metal-free organocatalytic approaches using hypervalent iodine (III) reagents have been reported by Muniz and Johnston,5 including a catalytic variant used for diamination of stryrenes.6 Very recently Denmark has published a catalytic enantioselective syn-diamination of alkenes using a Se(II) / Se(IV) redox cycle.7Electrochemical methods have also been explored and are summarised in recent paper by Xu et al.8
The advantage of the Minakata method, in addition to negating the use transition metals, is that it enables stereospecific access to both syn– and anti-1,2-diamines from both acyclic E– and Z– as well as cyclic alkenes.
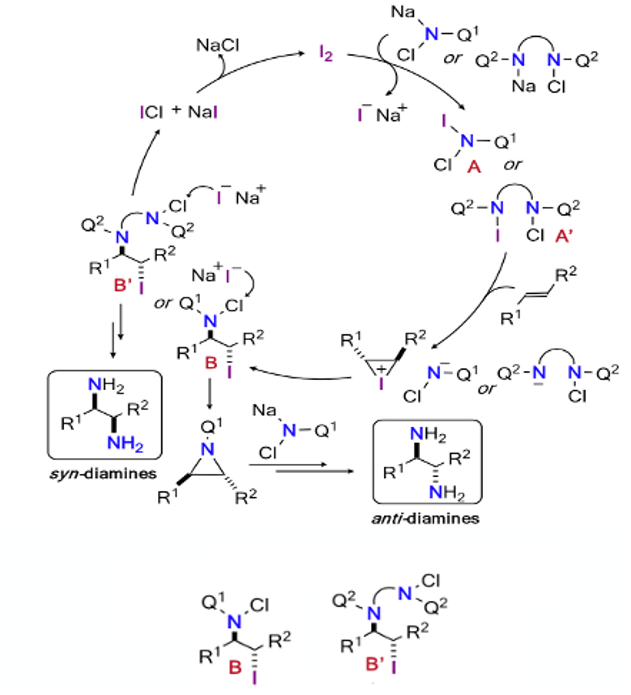
The key to this approach is the utilization of a N-halogen bond to transfer a nitrogen atom to the olefin substrate. Historically, the team had shown that iodine-catalyzed aziridination of alkenes using chloramine-T (N-chloro-N-sodio-p-toluenesulfonamide, Figure 3) was an efficient way of making these small heterocyclic rings.9 In an extension of this work, ring-opening of the strained aziridines with a nitrogen source (the chloramine salt) would generate diamines with anti– relative stereochemistry. The proposed mechanism is shown in Figure 4. Aziridination proceeds through the anti– iodoaminated intermediate (B) that comes from the N-chloro-N-iodo amide species (A). To divert the process to a syn– diamination, a species containing a pendant nitrogen source to essentially ‘invert’ the anti– stereochemistry would be required (B’). Both intermediates would originate from the three-membered iodonium generated from reaction of the olefin with A (or A’).
Guided somewhat by choice of a suitable N-protecting group that’s easily removed, o-nitrobenzenesulfonamide (nosylamide-Ns) was chosen and screened against a range of halogen-containing oxidants with the ultimate aim of in situ generation of chloramine-Ns using nosylamide. The screen consisted of treating phenyl-1-butene with nosyl-amide in the presence of I2 as the catalyst with the chlorinating agent. Solid sodium hypochlorite pentahydrate (NaOCl·5H2O) gave full conversion to the (bis-Ns-protected) diamine without any of the corresponding intermediate aziridine being detected. This is an oxidant I’ve not used in the solid form before. Aqueous hypochlorite (bleach) on the other hand is a different storey. Kirihara published a review on its use as an environmentally benign oxidant for organic synthesis is Organic Process Research and Development in 2017.10 He claims its non-explosive and stable on cold storage below 7°C. It’s been known in the literature since 1919 and was first supplied on industrial scale in 2013 by Nippon Light Metal Co., Japan. Several vendors now list it as a commercially available reagent. As of March 2021 the review has been viewed almost 13K times with 27 citations, with an altmetric score of 18.
The nitrosulfonamide group is easily deprotected via Meisenheimer complexes formed upon treatment with thiolates in DMF at room temperature according to the procedure developed by Fukuyama.11
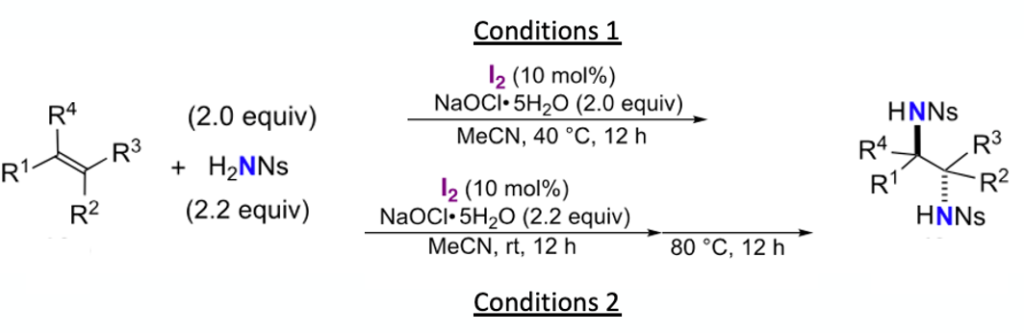
The conditions (1 or 2) depend on the alkene substrate and are shown in Figure 5. Alkene substitution pattern and steric hinderance in the intermediate azetidine seems to dictate which conditions are successful, with terminal olefins or ‘tied back’ cyclic olefins reacting under milder reaction conditions (1) than disubstituted alkenes. Olefin geometry (E/Z) in this case dictates the relative stereochemistry of the diamine products. Dip into the paper for examples if you’re interested.
Since ring-opening of the aziridine intermediate was rate limiting for formation of diamines from di-substituted olefins (requiring additional heating at a higher temperature) addition of a second nitrogen nucleophile (NH2Boc) was possible, giving the orthogonally protected Ns and Boc- diamine: E-2-octene giving the Ns/Boc anti-diamaine in 85% yield. Each orthogonal protecting group could be individually deprotected to the corresponding mono-protected compound- a useful addition to the methodology.
In the case of cyclic olefins, in which the double bond geometry is fixed, 1,2-anti-diamination was predicted (and observed). In order to access Syn– diaminated products from cyclic alkenes an alternative ‘di-nitrogen’ reaction manifold (as depicted in Figure 4) was explored.
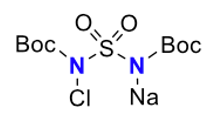
Continuing the sulfamide theme, chloramine BBS (N,N′-bis(tert-butoxycarbonyl)sulfamide, Figure 6))- a compound yet to find application in organic synthesis, was found to be effective in diethyl ether solvent with a slightly higher iodine catalyst loading (15mol%) (Figure 7). No anti– adducts were detected in the examples presented in the paper. A one-pot syn– diamination was possible via in situ preparation of chloramine-BBS from N,N′-bis(tert-butoxycarbonyl)sulfamide using NaOCl2.5H2O. A solvent swich from acetonitrile to diethyl ether was required for the reaction to work.
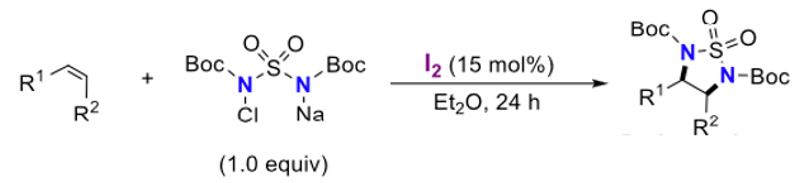
This chemistry appears to be neat and efficient- the only byproducts being NaCl and H2O. The safety and thermal hazards would obviously to be investigated if the chemistry were to be used at scale- N-halogen (and O-halogen) intermediates are potentially very energetic species. A review on halonium catalysis has just been published by Li in Synlett.12 I think we can expect to see a lot more examples of this approach in the coming years. So the I’s have it! See you next time.
References:
- Diastereodivergent intermolecular 1,2-diamination of unactivated alkenes enabled by iodine catalysis: S. Minakata et al, J. Am. Chem. Soc. 2021, https://dx.doi.org/10.1021/jacs.1c00228
- Vicinal diamino functionalities as privileged structural elements in biologically active compounds and exploitation of their synthetic chemistry: G. Li et al, Biol. Drug Disc. 2006, 67, 101-114; D. Methods for direct alkene diamination, new and old: Wardorp et al, Tetrahedron 2012, 68, 4067-4105.
- Catalytic asymmetric dihydroxylation: K. B. Sharpless et al, Chem. Rev. 1994, 94, 2483-2547.
- Catalytic, asymmetric osmium-free dihydroxylation of alkenes: C. Wang et al, Synthesis 2021, 53, 1229-1236.
- Enantioselective metal-free diamination of styrenes.: K. Muniz et al, Angew. Chem., Int. Ed. 2011, 50, 9478−9482; Oxidative inter-/intramolecular alkene diamination of hydroxy styrenes with electron-rich amines: J. Johnston et al, Org. Lett. 2015, 17, 2558−2561.
- Catalytic asymmetric diamination of styrenes: K. Muniz et al, J. Am. Chem. Soc. 2017, 139, 4354-4357.
- Catalytic, enantioselective syn-diamination of alkenes: S. Denmark et al, J. Am. Chem. Soc. 2019, 141, 19161–19170.
- Practical and stereoselective electrocatalytic 1,2-diamination of alkenes: H. Xu et al, Nat Commun. 2019, 10, 4953
- Iodine-catalyzed aziridination of alkenes using chloramine-T as a nitrogen source: S. Minakata et al, Tetrahedron 1998, 54, 13485-13494.
- Sodium hypochlorite pentahydrate crystals (NaOCl.5H2O): A convenient and environmentally benign oxidant for organic synthesis: M. Kirihara et al, Org. Process Res. Dev. 2017, 21, 1925-1937.
- 2- and 4- nitrobenzenesulfonamides: Exceptionally versatile means for preparation of secondary amines and protection of amines: T. Fukuyama et al, Tett. Lett. 1995, 36, 6373-6374.
- Halonium catalysis: an underutilized and underexplored catalytic concept in olefin functionalisations: W. Li et al, Synlett 2021, 32, 539-544.








