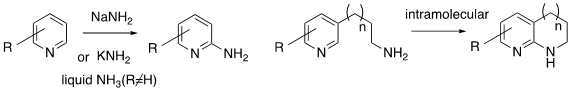

2-Aminopyrides are useful synthetic intermediates and pharmacophores in a number of drugs.[1] The Chichibabin reaction, developed in the 1900’s gives a direct method for the amination of pyridine and pyridine derivatives (azines and azoles) using sodium or potassium amides. Intramolecular amination reactions are also known. The reaction can be run either at high temperature in a solvent resistant to NaNH2 (aryl compounds, mineral oil, dialkylamines or neat), or at low temperature in liquid ammonia using the more soluble KNH2. The latter method is homogeneous and can be used for temperature sensitive substrates such as diazenes and triazines. The low temperature method does not, however, work for unactivated pyridines. Addition of an oxidant (e.g. KMnO4) to the low temperature process facilitates removal of hydride ion.
The mechanism is shown below and is formally nucleophilic addition of an amide anion (NH2–) to the aromatic compound with loss of hydride (H–). Complexation of the pyridine nitrogen atom with sodium (or potassium) ion promotes addition of the amide anion by increasing the positive charge at C2. The resulting sigma-complex aromatises with loss of hydrogen gas and formation of the sodium salt which is quenched by water on work-up.2-Aminopyrides are useful synthetic intermediates and pharmacophores in a number of drugs.
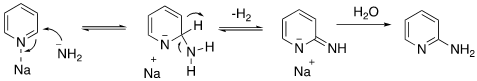
[1] Int J Pharm Bio Sci 2017 Apr ; 8(2): (P) 338-355








