I always look forward to publication of the annual review synthetic approaches to the new drugs approved for the preceding year. In some ways its bitter sweet since it acts as a reminder of how difficult drug discovery and development is and how the large volume of molecules that start their journey never actually make it through development to the patients that need them. There are, of course many reasons for this- toxicity, lack of target engagement or efficacy, poor exposure or a bad choice of target/poorly understood biological mechanism. Not all of the reasons are scientific- business and science are not always good bedfellows. The ultimate aim for any commercial enterprise is to make money for its shareholders and sometimes this results in abrupt termination of projects or whole areas of research. I’m reminded of the resent news that Novartis is moving out of the anti-infectives field. A huge blow to the hundreds of scientists working on new therapies. And this in spite of the World Health Organization’s warning that antimicrobial resistance “is one of the biggest threats to global health, food security and development today”.
Anyway- I digress. The annual publication of the structure and synthesis of new NCE’s was started 15 years ago by Jin Li and Kevin Liu from Pfizer, publishing in mini reviews in medicinal chemistry.[1] The review moved to its new home in the Journal of medicinal chemistry, the ACS flagship journal for drug discovery, a year or two ago. Several authors have come and gone over the years. New formulations, polymorphs or other re-hashes of existing medicines don’t count (rules of the house). The synthetic routes highlighted are, as far as I’m aware, not necessarily the manufacturing route or even a scalable route- it just anchors the reader to a synthetic approach that has been used to make the drug, most likely disclosed in the primary literature or early patent filings. As most process chemists are aware the synthetic route goes through many incarnations before a final manufacturing process is established and approved by the regulators. The synthetic route used to make material during the lifecycle of the drug depends on the stage of development and material requirements. The chemical development and scale-up course run by Scientific Update gives much more detailed information and case studies for those interested in learning more. The annual review also gives information on the biological target
I always recommended that both medicinal chemists and process chemists who are starting their career in the pharmaceutical industry look at the structures and synthetic routes to give them ideas and possibly help them with challenges they may be facing in their own chemistry. In the words of Sir James Black, winner of the 1988 Nobel prize in medicine “the most fruitful source of a new drug is to start with an old drug”
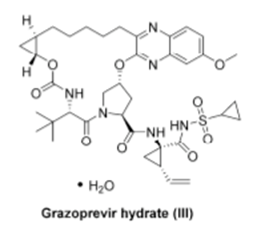
I chose the structure of Grazoprevir, a protease inhibitor for HCV therapy, as a picture for the article for no other reason than I liked the complexity and the challenging chemistry. Happy reading.
[1] mini reviews in medicinal chemistry 2002, 4, 207








