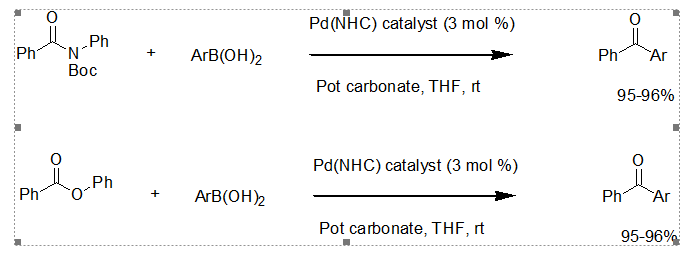
The Suzuki-Miyaura cross coupling reaction has been widely used in industry for C-C bond formation but has not successfully been applied to unactivated substrates such as amides and esters, in which the C-N and C-O bonds are cleaved. This recent report from scientists in Wroclaw (Poland), Beijing and New Jersey gets around the normally slow oxidative addition of the substrate amide/ester to the metal, which in the past has resulted in poor selectivity when high temperature are used to speed the process. By using Pd-NHC catalysts, common esters and amides can be coupled at room temperature. The disadvantage for industry is having to use phenyl esters and Boc- protected amides.
Lei et al, Chemical Science, 2017, 8, 6525.