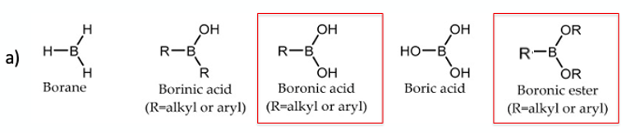
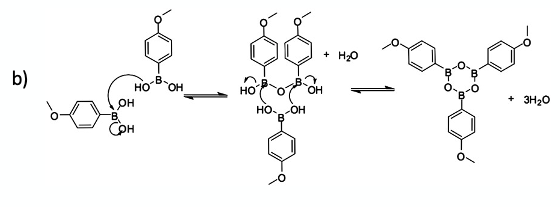
I can’t begin to count the number of aryl boronic acids I’ve made and/or worked with over the years. These versatile intermediates will forever be part of the synthetic organic chemist’s toolbox. You would be hard pushed to find an organic chemist that hasn’t run a Suzuki- Miyaura cross coupling reaction at some stage in their career- or at the very least written a synthetic scheme or worked through the mechanism in an exam paper. Boronic acids have even found their way into marketed drugs and clinical development candidates.1 From a handling and isolation perspective, boronate esters are frequently utilised and used as surrogates directly in cross coupling chemistry (Figure 1a). The free boronic acids tend to be somewhat temperamental- with C-B bond stability issues, high polarities (hindering chromatographic isolation), and the propensity to spontaneously dehydrate to give boroxines (Figure 1b).
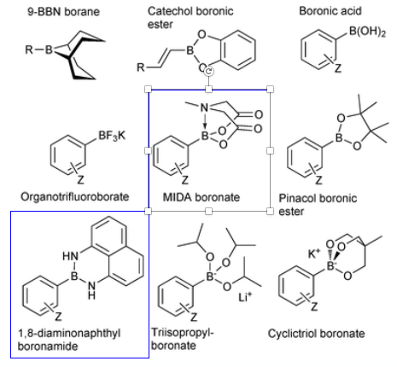
Pinacol boronates are the go-to intermediates for Suzuki cross-coupling reactions. They are easily prepared directly from the arylboronic acid or by metal-catalysed coupling of a suitably substituted aryl halide (predominantly bromide)) with bis(pinacolato)diboron. A comprehensive review was published by Marder and Westcott in 2016.2a A good review of C-H activation for the construction of C-B bonds was published by John Hartwig in 2010.2b Many pinacol boronates can be purified on silica, however prolonged contact with this separation medium results in hydrolysis and subsequently poor recovery. In my experience flashing crude material rapidly through a silica plug and slurrying the isolated product in heptane generally gives a good recovery of reasonable quality material. HPLC analysis of these intermediates can be a challenge. Another purification approach reported by Isobe involves pre-treating the silica gel with boric acid- something I must admit I’ve never tried.
A notable innovation in this area by Martin Burke was the development of the trivalent N-methyliminodiacetic acid (MIDA, Figure 2) ligand as a boronic acid “protecting group”. Complexation with the ligand rehybridises the boron centre from sp2 to sp3 and attenuates the palladium transmetalation step in cross-coupling reactions.4 1,8-Diaminonapthalene aryl boron amides have also been reported by Yoshida (among others) ((Figure 2). These are less attractive than MIDA boronates since they require strongly alkaline conditions for direct coupling.5a A comprehensive review of boron reagents used in Suzuki-Miyaura cross-coupling reactions was published by Guy Lloyd-Jones in 2014.5b
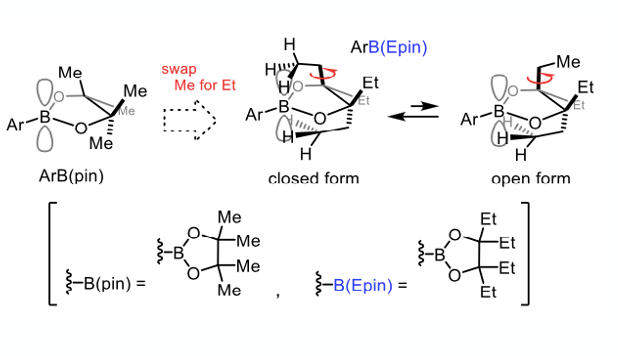
You’d be forgiven for thinking boron ester chemistry has nowhere else to go, having reached its pinnacle (if you’ll pardon the pun). In spite of the methyl, ethyl, butyl, futile- adage familiar to medicinal chemists (i.e. refraining from the diminishing returns inherent in synthesising close analogues), Ikawa and Akai have pushed beyond pinacol to 1,1,2,2-tetraethy glycol esters (ArB(epin)s, Figure 3)- apparently an island of stability in a sea of possible alky esters (and amides).6 These esters show enhanced stability over the pinacol system enabling purification over silica gel and good recovery from the work-up.
It’s not clear if there was a design element to this chemistry- despite the authors claiming there was. It’s not uncommon for authors to embellish their results with a retroactive design approach. 3,4-Diethyl-3,4-hexandiol (CAS #6931-71-1) is not easy to come by, suggesting there may well have been some prior rationale. No additional “methyl, ethyl, butyl, futile “ SAR is presented. The rationale is that the four ethyl groups of the boronate were designed to spatially protect the empty orbital on boron via a dynamic conformational equilibration, with the “closed form“ providing kinetic stability (Figure 3).
The epin boronate esters described in the paper were prepared using similar chemistry to that used for the corresponding pinacol analogues (Figure 4). Plenty of diversity in the structure of the ArB(epin)s is disclosed in the paper and supplementary information. Presumably commercially available epin diol is prepared by a pinacol coupling reaction.7 Hindered diols can undergo dehydration with rearrangement under acid catalysis (the well-known pinacol rearrangement). I guess boronic acids are not acidic enough to promote this reaction vide infra.
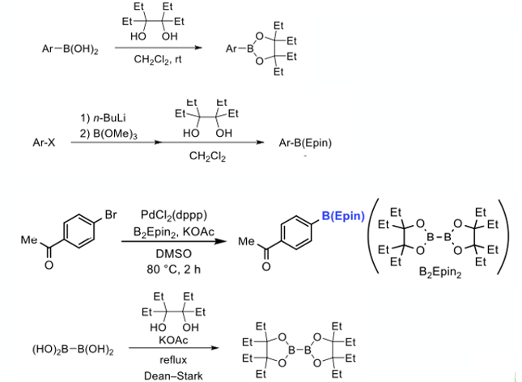
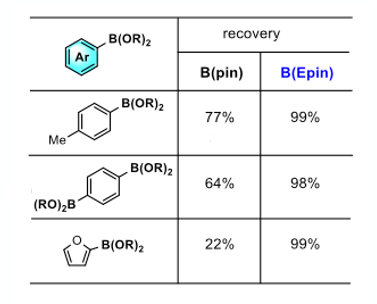
So now to the acid test as it were. ArB(epin) and ArB(pin) ester derivates of three structural different arylboronic acids were compared head-to-head, looking at recovery on silica gel upon eluting with ethyl acetate (10cm x 25mm i.d). The epin derivatives gave quantitative recovery with the corresponding pin systems struggling (Figure 5).
TLC analysis of the boronates reflected these findings- ArB(pin) derivatives showing extensive streaking/decomposition whilst the corresponding ArB(epin) systems ran as discreet spots (5-10% EtOAc/hexane).
Perhaps more importantly the ArB(epin)s performed well in direct Pd mediated cross coupling with aryl bromides under typical Suzuki conditions (Sphos ligand, Pd(OAc)2, K3PO4, toluene/water, 110°C, 24hrs).
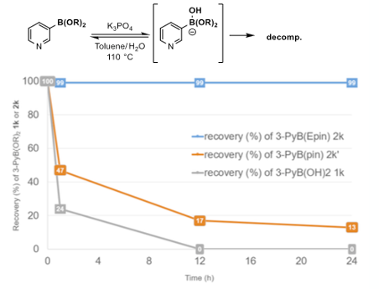
An interesting finding reported in the paper was the apparent increased stability of 3-pyridylboronate(epin) v’s both the pinacol- ester and the free boronic acid in the presence of aqueous base at high temperature. The 2-pyridyl systems are known to be unstable with respect to protodeboration, however the issue can be addressed nicely using the MIDA boronates.8a The mechanism of protodeboration of heteroaromatic boronic acids has been investigated by Guy Lloyd-Jones.8b The 3-pyridyl isomer, however, is somewhat more stable. Three separate experiments were conducted: heating parent 3-pyridy boronic acid, epin boronate and pinacol boronate in aqueous K3PO4 / toluene at 110°C. The Boronic acid experiment showed complete decomposition of the intermediate after 12 hours. In the case of pinacol boronate, low levels remained after the same time period. With the epin ester the intermediate was completely unscathed (Figure 6).
Now my understanding of the mechanism of the Suzuki- Miyaura reaction using boronate ester intermediates was that hydrolysis to the boronic acid was required before transmetalation with palladium. However, an interesting paper published recently by Scott Denmark in which he used structural, computational and kinetic investigations to look at the role of boronate esters in direct transmetalation suggests that boronic esters can transmetalate directly without prior hydrolysis.9 This would confirm the observation that the 3-pyridyl(epin) was active in cross coupling chemistry.
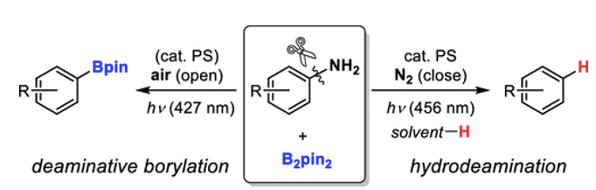
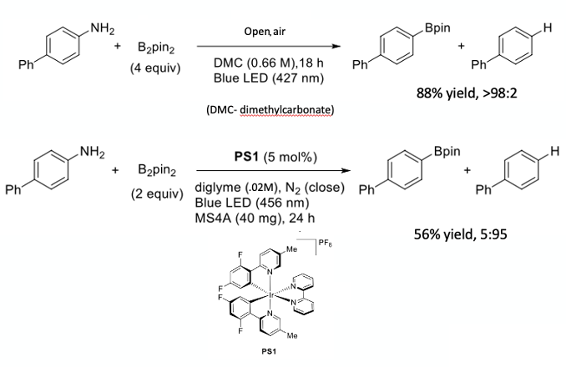
Finally, another interesting paper relating to bis(pinacolato)diboron by Sekine et al just caught my eye.10 The paper describes a photoinduced divergent deaminative borylation and hydrodeamination of primary aromatic amines using bis(pinacolato)diboron. Depending on reaction conditions either the boronate ester or the reduced aniline can selectively be produced (Figure 7). Hydrodeamination is shown to proceed via hydrogen atom transfer between the corresponding aryl radical and an ethereal solvent (diglyme being optimal) (Figure 8).
A few interesting things to think about here. I don’t believe that ArB(epin) esters will ever replace the well-established pinacol-esters, however it does show that methyl, ethyl butyl futile can very occasionally take us to interesting places.
See you next time.
References:
- Boronic acids and their derivatives in medicinal chemistry: synthesis and biological applications: M. P. Silva et al, Molecules 2020, 25, 4323.
- a) Diboron(4) compounds: from structural curiosity to synthetic workhorse: T. Marder et al, Rev. 2016, 16, 9091-9161; b) J. Hartwig et al, Chem. Rev. 2010, 110, 890-931.
- A facile chromatographic method for purification of pinacol boronic esters: H. Isobe et al, Lett. 2012, 41, 972-973.
- A simple and modular strategy for small molecule synthesis: iterative Suzuki−Miyaura coupling of B-Protected haloboronic acid building blocks: M. Burke et al, J. Am. Chem. Soc. 2007, 129, 6716.
- a) Direct Suzuki-Miyaura coupling with naphthalene-1,8-diaminato (dan)-substituted organoborons: H. Yoshida et al, ACS Catalysis 2020, 10, 346-35; b) Selection of boron reagents used in Suzuki-Miyaura coupling: Lloyd-Jones et al, Chem. Soc. Rev. 2014, 43, 412-443.
- Aryl boronic esters are stable on silica gel and reactive under Suzuki-Miyaura coupling conditions: S, Akai, T. Ikawa et al, Org. lett. 2022, 24, 3510-3514.
- “New” reagents for the “old” pinacol coupling reaction: T. Wirth Chem., Int. Ed., 1996, 35, 61-63.
- a) A general solution for the 2-pyridyl problem: M. Burke et al, Angew. Chem. Int. Ed. 2012, 51, 2667-2672; b) Protodeboronation of heteroaromatic, vinyl, and cyclopropyl boronic acids: pH–rate profiles, autocatalysis, and disproportionation: G. Lloyd-Jones et al, J. Am. Chem. Soc. 2016, 138, 9145–9157.
- Elucidating the role of the boronic esters in the suzuki–miyaura reaction: structural, kinetic, and computational investigations: S. Denmark et al, J. Am. Chem. Soc. 2018, 140, 12, 4401–4416.
- Photoinduced divergent deaminative borylation and hydrodeamination of primary aromatic amines: K. Sekine et al, ASAP Org. Lett. 2022, https://doi.org/10.1021/acs.orglett.2c01663








