Last week I was in Madrid running our chemical development and scale up course. I met a great group of people, one of whom worked at Dynamit Nobel. During a coffee break we struck up a conversation about (of all things) azides, in particular sodium azide. Fairly quickly we both discovered that we had no idea how it’s made industrially. Very carefully would be my somewhat cynical suggestion. Curiosity sufficiently piqued, I thought I would look into it. This post is a short summary of my findings.
A quick google search revealed references to the use of sodium azide as a precursor to organic azides and for synthesis of lead azide (used as a detonator to initiate secondary explosives).1 The 2022 chemistry Nobel prize is based on azide “click” cycloaddition reactions. The organic azide substrates used in this chemistry will ultimately have come from sodium azide at some point in their synthesis.2 I found a lot more recent hits than I had expected. It turns out that NaN3 is used (at a very low concentration) in the liquid buffer contained in (at least some) Covid-19 rapid antigen tests.3 Its bacteriostatic (halts bacteria growth without killing them- the opposite to bactericidal). The azide works by inhibiting cytochrome oxidase in gram–negative bacteria. However, some gram-positive bacteria (streptococci, pneumococci, lactobacilli) are intrinsically resistant. Cell division arrest is believed to occur by formation of a complex between a haem-A iron ion and a copper B-ion located within the O2 reduction site.4 NaN3 is often used to prevent aqueous media in research biology labs from growing bugs.4 The high aqueous solubility (40g/100ml at 17°C) lends itself well to this application. The expected global demand for NaN3 is expected to boom. I couldn’t find the expected market size (unless I paid a few thousand dollars for a marketing survey!). In 2010 the global production was around 1000 t/a. This number today is probably significantly higher. Human beings being somewhat unpredictable, unsurprisingly a few people (mostly children) have tasted or drunk the buffer. US poison centres have fielded questions from concerned parents.5 Is it a very toxic compound,6 though relatively benign at the concentration used in the buffers. Incidents have also been reported in US and Canadian hospital labs with technicians disposing of aqueous sodium azide (presumably present in aqueous media or assay samples) through the sink drain. The azide can react with metals in the waste pipes (particularly copper) and generate shock-sensitive metal azides. These materials can accumulate over time and cases of people unblocking drains triggering an explosive decomposition have been documented.7 Metal azide salts, particularly transition metal salts, are usually highly explosive, shock-, friction-, and static-sensitive materials. Copper (II) azide in particular is very bad actor.7b
Another legacy application of sodium azide is its use in inflation of airbags (in cars and aeroplane escape slides).8 Developed in the 1960’s, the original design involved an event- triggered explosive decomposition of NaN3 rapidly generating nitrogen and filling the bag. These early devices have now been superseded and use other materials such as ammonium nitrate.8 A detailed study of the decomposition of sodium azide by DSC was published by Potvin in 1972, together with some interesting findings on inhibition of decomposition by water.9
Sodium azide has been used in the synthesis of many drug molecules, including construction of the tetrazole ring in the Sartan family of anti-hypertension drugs via nitrile cycloaddition.10 Figure 1 shows some of the frequently used synthetic methods employing NaN3.
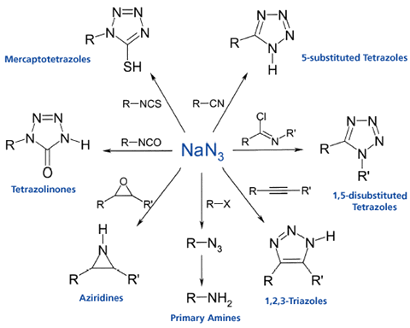
Before I come to the manufacturing route a word on the risks of generating hydrazoic acid. Addition of acids (or even water) to sodium azide (or azide containing waste streams) generates HN3 – an extremely shock sensitive explosive. The detonation speed of HN3 is of the order of 8000m/s. For reference, TNT comes in at 7000 m/s and nitroglycerine 7800 m/s. Fractions of a millilitre of HN3 can easily destroy a fume hood. Chemical processes that generate hydrazoic acid need extensive safety testing. A single drop of HN3 falling into a reaction mixture from a condenser can trigger a violent explosion. A recent analysis by Treitler and Leung discusses the risks and dangerous of working with azides or processes that can generate hydrazoic acid.11 I would highly recommend anyone working with sodium azide read this article. And then read it again.
Industrially, sodium azide is made using a two-step process developed by Johannes Wislicenus in 1892.12 The first stage involves generation of sodium amide at 350°C by reaction of molten sodium with ammonia in a steel reactor (Figure 2). Any water present in the system and its really game over.
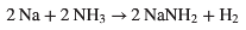
The molten sodium amide is then converted to sodium azide using nitrous oxide at 230°C in a nickel reactor (Figure 3). The nitrous oxide is usually generated by thermal decomposition of ammonium nitrate. The ammonium nitrate comes from reaction of the ammonia generated in stage 2 with nitric acid. Nothing is ever wasted on an industrial scale.
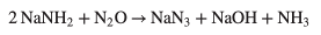
Purification involves dissolving in water, a clarifying filtration and evaporation. This generates a crystalline solid that is dried at 110°C. The overall yield (based on sodium) is around 90%. Any residual sodium azide can be decomposed using nitrous acid (generated from sodium nitrite and nitric acid). This reaction is also used to destroy excess sodium amide used in general synthetic chemistry (for example synthesis of tetrazoles from nitriles). The Wislicenus process has been developed and run continuously.13
A variation of this process, reported by Degussa, uses sodium monoxide in combination with nitrous oxide and ammonia (Figure 4) in a single step reaction. The advantage here is that no gases are generated and the process can be run at higher pressure. The sodium hydroxide by-product is removed using liquid ammonia or ethanol. Another one-step process is fusion of sodium nitrate with sodium amide at 170°C.12
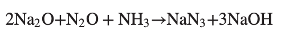
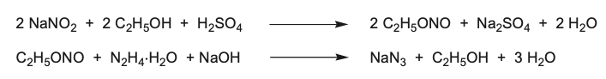
Curtius (he of the very useful rearrangement process) and Thiele developed an alternative diazotisation approach used for lab-scale synthesis (Figure 5). As far as I’m aware this is not used at industrial scale.1 Reaction of ethanol with sodium nitrite generates the nitrite ester. Treatment of this intermediate with hydrazine produces sodium azide and ethanol (Figure 5). Using hydrazine at scale introduces additional thermal saftey hazards.
So this is how it’s done. Processes used for industrial manufacture of the reagents we use routinely are often taken for granted. Sometimes we can learn a great deal by taking the time to look at these and implement some of the findings in our own work.
See you next time.
References:
- Large-scale preparation and usage of azides: J. Haase Organic Azides: Syntheses and Applications Ed. Stefan Bräse and Klaus Banert, 2010 Wiley, ISBN: 978-0-470-51998-1
- Introduction: click chemistry: N. Devaraj & M. Finn, Chem Rev. 2021, 121, 6697-6698
- Human toxicity from COVID-19 rapid home test kits: K. Johnson-Arbor et al, Am. J. Emerg. Med. 2022, 57, 215-216
- Studies of the effect of sodium azide on microbic growth and respiration: H. Lichstein et al, Journal of bacteriology1944, 47, 221-230
- Sodium azide poisoning: a narrative review: S. Satomi et al, Clin. Tox. 2021, 59, 683-697
- Sodium azide: https://pubchem.ncbi.nlm.nih.gov/compound/Sodium-azide
- a) Current intelligence bulletin 13: explosive azide hazard: CDC, 1976, Link ;b) Thermal decomposition of certain inorganic trinitrides: A. Hitch, Am. Chem. Soc. 1918, 40, 1195
- What chemicals make airbags inflate, and how have they changed over time?: B. Halford C&N News Nov 21, 2022
- A study of the decomposition of sodium azide using differential thermal analysis: H. Potvin et al, Can. J. Chem. 1973, 51, 183-186
- A review on synthesis of antihypertensive sartan drugs: N. Pati et.al International Journal of Pharma Research & Review 2014, 3, 46-56
- How dangerous is too dangerous? A perspective on azide chemistry: D. Treitler & S. Leung, Org. Chem. 2022, 87, 11293-11295
- Hydrazoic acids and azides: S. Brase et al, Ullmans encyclopaedia of industrial chemistry, 2015, 1002/14356007.a13_193.pub2
- Process for the continuous production of sodium azide: US5176895; CA2046385








