Phenols and anilines are two of the most important building blocks in industrial chemical manufacturing. Any process capable of converting one into the other is going to generate a lot of interest, and a paper by Shi and co-workers does just that.1
Direct conversion of an aniline to a phenol is a relatively straightforward transformation via the diazonium salt in a Sandmeyer process. Phenols on the other hand, with a Ph-OH bond dissociation energy of >450 kJ/mol, preclude transition-metal catalysed cross-coupling reactions and C-N bond formation. Indirect activation with a suitable sulfonate group (typically trifluoromethylsulfonyl) facilitates oxidative addition to a metal such as Pd or Ni, and anilines are easily obtained by reductive elimination in a Buchwald-type reaction.2 Other approaches include Smiles-type rearrangement,3b and deoxygenative N-centred radical substitution.3b
The paper by Shi describes a reaction driven by condensation of an amine with the keto-tautomer (dienone form) of a phenol- a species that is thermodynamically unstable with respect to the fully aromatic system- the species that is exclusively present in solution (Figure 1).4 By comparison, acetone has a very low equilibrium concentration of the enol form (~1×10-7 %)! What’s needed is a way to drive the phenol/dienone equilibrium from left to right as it were.
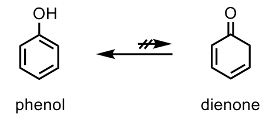
This approach has been explored by other groups using several different strategies including dearomatization/dehydrogenation using heterogeneous catalysis to convert phenols to anilines,5a and oxidative dearomatization, condensation and redox isomerization.5b
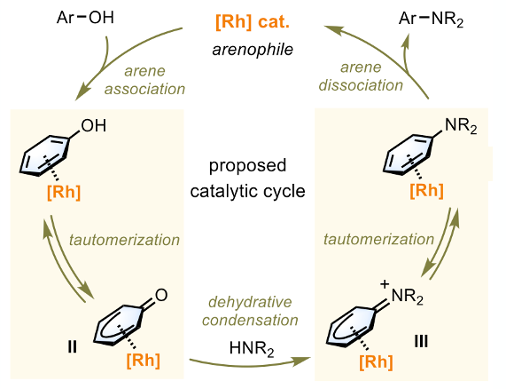
It’s known generally that Lewis acids can drive a keto-enol equilibrium toward the enolate form. The key to the Shi approach was to explore if the same protocol could be used to drive the phenol/dienone to the keto-form (Figure 1) enabling a dehydration/amination reaction. To this end a series of arenophilic Rh(III) complexes were screened, focusing on those capable of forming π-complexes with the deprotonated phenol and, more importantly, a transient η5phenoxo complex (Figure 2, II). The idea was that amine condensation would then give an imine (Figure 2, III) and ultimately the aniline via tautomerisation. An aryl bis-trifluoromethyl-Rhodium complex proved the best catalyst for achieving this goal (Figure 3). The reaction mechanism was confirmed using deuterium labelling experiments together with time course experiments that showed smooth conversion of phenol to aniline without imine or ketone intermediates being observed (using the reaction of 4-methylphenol with piperidine as a general example).
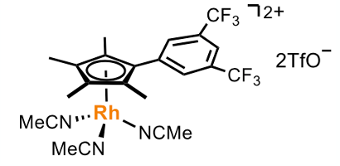
Standard generic conditions for the substrates examined in the paper were 2.5mol% catalyst, 10mol% Na2CO3, 4Å molecular sieves, n-heptane, 120°C (up to 140°C). Substrate scope was broad with respect to both the phenol and the amine component, and the system showed good functional group tolerance. Several of the yields are on the low side (40-50%), however I’m sure optimisation of reaction conditions for a specific substrate of interest would give a better yield- something process chemists often rely upon.
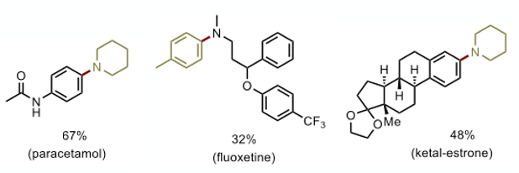
A couple of notable examples- conversion of paracetamol to the piperidine derivative (67% yield), N-arylation of fluoxetine (32%) and formation of the piperidine analogue of ketal estrone (48%) (Figure 4). These demonstrate an opportunity for late-stage functionalisation.
Overall an interesting reaction requiring no pre-activation of the phenol in a redox neutral process with water as the by-product. I would have liked to have seen lower catalyst loadings- rhodium is a particularly expensive metal, however further ligand modifications may bring the S/C ratios down.
This is one of those papers where one feels certain it must have been done before. Just goes to prove that nothing should be taken for granted and there’s always holes in our understanding that can be filled by those willing to rise to the challenge.
See you next time.
References:
- Catalytic amination of phenols with amines, H. Shi et al, J. Am. Chem. Soc. 2022, 144, 1144-1151.
- Palladium-catalysed amination of aryl triflates, S. Buchwald et al, J. Org. Chem. 1997, 62, 1264-1267.
- a) One-pot conversion of phenols to anilines via Smiles rearrangement, Y. Xie et al, Tet. Lett. 2013, 54, 5151-5154; b) Formal aniline synthesis from phenols through deoxygenative N-centred radical substitution, V. Schmidt et al, Chem. Euro. J. 2019,67, 15267-15271.
- Naphthols on the other hand can tautomerize to the enones because the energy barrier to dearomatization of condensed rings is relatively low.
- a) Formal direct cross-coupling of phenols with amines. C. Li et al, Angew. Chem., Int. Ed. 2015, 54, 14487-1449; b) Unified synthesis of 1,2-oxy-aminoarenes via a bio-inspired phenol-amine coupling, J. Lumb et al, Chem. 2017, 2, 533-549.








