Normally when one chlorinates a phenol one would expect to see perhaps 8:1 to 10:1 regioselectivity in favour of the para-isomer, but in this paper using N-chlorosuccinimide as the chlorinating agent, using the correct thiourea catalyst can give up to much better regioselectivity for either the o-chloro or p-chloro isomer depending on the thiourea catalysts used. With catalyst 6, selectivities of up to 10:1 in favour of the o-chloro can be obtained, whilst with catalyst 7 the p-chloro isomer is the major product with regioselectivity up to 20:1. Reactions were run in CDCl3 to allow easy nmr analysis of the isomer ratios.
Scheme: Regioselective chlorination of phenols
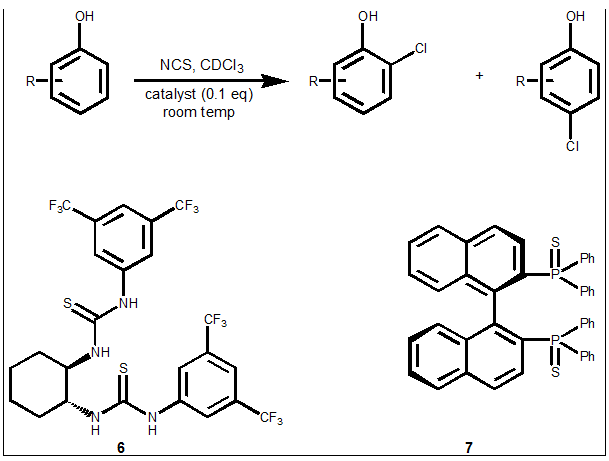
Further work is ongoing to try and elucidate the reaction mechanisms.
S.M. Maddox, A.N. Dinh, F. Armenta, J. Um, and J.L. Gustafson,* Org. Lett., 2016, 18, 5476-5479.








