Figure 1
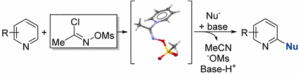
I guess to qualify as a reagent of the month the material has to fulfil certain criteria and commercial availability, though not essential, is very desirable. N-((Methylsulfonyl)oxy)acetimiodyl chloride (henceforth known, at least by me, as Fier’s reagent) is now available to buy from Sigma Aldrich (CAS: 1433887-06-9). The compound is an air and moisture stable crystalline solid and has utility as a bifunctional intermediate for pyridine elaboration.[1] Designed and published last year by Patrick Fier from Merck Rahway it enables the mild, nucleophilic functionalization of pyridines in the C2 position (J. Am. Chem. Soc. 2017, 139, 9499).[2] This methodology is an improvement over the traditional N-oxidation/activation approach and avoids per-oxidation of the pyridine nitrogen atom and its inherent safety risks.
Equilibrium reaction of a suitable pyridine substrate with the α-chloro-O -methanesulfonyl aldoxime (to generate the intermediate pyridinium hydrochloride salt, Figure 2) requires a stochiometric amount of NaOTf (1.2 eqv, CH3CN, 80°C, 1-2hrs) to drive the forward reaction by precipitation of NaCl. Several pyridinium salts disclosed in the publication were isolated and characterized by X-ray crystallography.
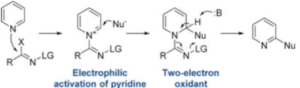
Figure 2
The paper describes and exemplifies direct cyanation of a range of substituted pyridines and the procedure demonstrated good functional group tolerance (including boronate esters, aldehydes, esters and halides) generating the 2-cyanopyridine derivatives in high yield (NaCN, Na2CO3, rt).[3] Of note is that many of these functional groups are reactive to conditions in which pyridine-N-oxide formation would normally be carried out. A mechanism is proposed in which irreversible cyanide addition occurs (confirmed by deuterium labelling) before deprotonation and formation of required 2-substituted product, with CH3CN, NaOMs and NaHCO3 generated as water-soluble waste products (Figure 2).
Preliminary results from reaction of the intermediate pyridinium salts with methoxide, Grignard, organozinc reagents and malonate anion are also presented and suggest that there is considerable scope for future innovation in this approach.
[1] It is made from acetaldehyde oxime by chlorination with NCS, followed by reaction with MsCl/Et3N.
[2] Pyridines are ubiquitous in the pharmaceutical and agrochemical industries, see J. Med. Chem. 2014, 57, 5845; De Novo synthesis of substituted pyridines Tetrahedron 2004, 60, 6043.
[3] Interestingly, Zn(CN)2 gives the 4-cyano derivative as the major product, NaCN and KCN both give the 2-cyano.








