Netarsudil- a ROCK kinase inhibitor marketed by Aerie pharmaceuticals as RhopressaTM– was recently approved by the FDA for ocular hypertension.1 An asymmetric synthesis is challenging due to the aryl group lowering the pKa of the α-hydrogen, rendering it very susceptible to racemization both during synthesis of the amino acid and subsequently amide coupling to an unreactive, poorly soluble amine nucleophile (6-aminoisoquinoline). Sturdivant et al describe an asymmetric synthesis of the β-amino acid starting material and conditions under which the amide coupling proceeds without racemisation using 2,2,2-Trichloro-1,1-dimethyl chloroformate (Figure 1)- an activating reagent not previously reported in a chiral amidation reaction (Synthesis 2019, 51, 953-959).
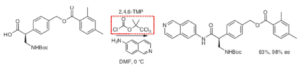
Figure 1: Netarsudil- non-racemising amide coupling
The acid was prepared in six steps from 2,4-dimethylbenzoate phenylacetic acid and the required (S)-stereochemistry was set using Evans chiral enolate chemistry. The β-amino arm was installed via direct alkylation of an enolate with N-Boc-1-aminomethyl benzotriazole (Figure 2).2
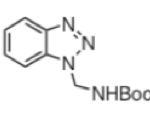
Figure 2: N-Boc-1-aminomethyl benzotriazole
Initial attempts to couple the acid with 6-aminoisoquinoline resulted in low yields and loss of enantiomeric integrity (HATU, EDC/DMAP, EDC/HoBt, PyAOP, CDI). The first hint of a possible way forward came from the use of ethyl chloroformate with a model substrate (N-Boc-2-phenylpropanoic acid, 96% ee, 40% yield).3 The main by-product was the ethyl ester and despite modifying the stoichiometry, base and temperature the low yield could not be improved upon. A screen of other chloroformates and anhydrides revealed that 2,2,2-trichloro-1,1-dimethyl chloroformate, a reagent not previously described as a coupling reagent, was successful both with the model substrate (>95% ee, 62% yield) and gratifyingly the desired substrate (63% yield, 98% ee). Presumably the urethane product (from attack of the amine on the ‘wrong’ carbonyl of the mixed anhydride) was not formed to any significant extent, however, this is not eluded to in the synthesis publication. A series of papers published by Novartis in OPRD some years ago discusses this problem when using iso-butyl chloroformate as the reagent for scale-up of an amide coupling.4 The benzoyl ester is also a potential reactivity hot-spot in this particular substrate I guess which might contribute to the modest yields.
Kinetic studies on the solvolysis of 2,2,2-trichloro-1,1-dimethyl chloroformate suggest the by-products are, as expected, HCl, CO2 and (non-nucleophilic) 1,1,1-trichloro-2-methyl propan-2-ol (c.f. EtOH from EtO(CO)Cl).5 The latter seems to me to be a potentially reactive by-product- the gem-dimethyl perhaps facilitating (via a Thorpe-Ingold effect) epoxidation and formation a PGI.
In the literature 2,2,2-trichloro-1,1-dimethyl chloroformate is most notably used as a protecting group.6 This paper highlights its use as a coupling reagent in sensitive amide bond forming reactions. I have personally carried out amide couplings where racemization of similarly labile substrates can be a real problem. I would certainly give this reagent a try if I were presented with similar challenges again. The described chemistry has apparently been run on kilo-scale too.7
References:
- Rho/Rho-associated kinase pathway in glaucoma (Review): Y. Zhong et al, Int. J. Oncology, 2013, 43, 1357-1367.
- Arvanitis et al, J. Chem. Soc., Perkin Trans. 1, 1998, 521.
- Large-Scale Applications of Amide Coupling Reagents for the Synthesis of Pharmaceuticals: J. Dunetz et al, Org. Process Res. Dev. 2016, 20, 140-177.
- Prashad et al, Org. Process Res. Dev. 2004, 8, 330; ibid 1999, 3, 409. See also A. Chaudhary et al, Org. Process Res. Dev. 2003,7, 888.
- Koh et al, Bull. Korean Chem. Soc. 2010, 31, 835.
- Wuts, P. G. M. Green’s Protective Groups in Organic Synthesis, 5th ed.; John Wiley & Sons: Hoboken, 2014, 928
- Sturdivant, J. M.; deLong, M. A.; Chambournier, G.; Pamment, M. G.; Fedij, V., (Aerie Pharmaceuticals Inc.) US Patent 9643927, 2017.








