I often stumble across interesting chemistry when I peruse my twitter feed. It’s not only rich in new chemistry highlights and ASAP links to journal articles but also chemists sharing their own experiences with both new and existing methodology- good, bad, and sometimes with pictures!- a dimension missing from just reading peer-reviewed journal articles and, for once, a positive aspect of social media. Trial by twitter. But any publicity is good publicity- right? Often people will also tweet links to new material submitted to the ChemRxiv pre-print server- raw manuscripts so fresh that the ink is still wet. Now admittedly this is crude material before purification (if you’ll pardon the pun)- it does need peer review- however, there’s something good about reading a paper before reviewer no.2 has rubbished it.
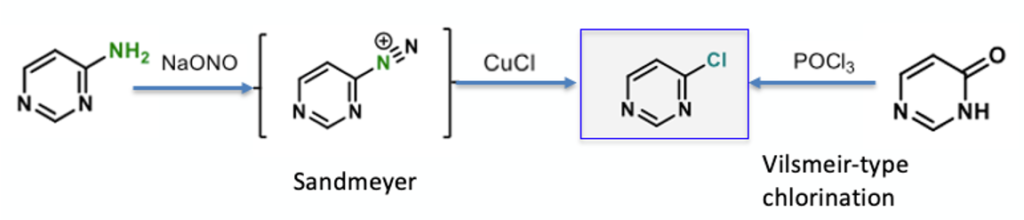
I came across a pre-print paper on ChemRxiv just recently on some work coming out of Josep Cornella’s lab at the Max-Plank Institute on deaminative chlorination of aminoheterocycles and it looks very interesting (posted online 23/3/2021).1 I’ve followed Cornella’s work on the use of pyrylium salts for C-N activation, in particular his paper on selective functionalization of aminoheterocycles published a couple of years ago in Angewandte Chemie.2 This latest offering describes the use of pyrylium salts to enable the NH2 group in aminoheterocycles to be converted directly to chlorides- essentially a Sandmeyer or Vilsmeir disconnection avoiding the use of hazardous diazonium salts or strongly oxidising chlorinating agents (Figure 1).3 Another indirect advantage over a sandmeyer process is avoiding the possible generation of nitrosamine impurities- a hot topic in the pharmaceutical industry right now.4 The conditions Cornella reports are mild, functional group tolerant and amenable to late-stage diversification (Figure 2). The resulting chlorides are masked modification handles that enable discovery chemists to rapidly explore SAR through coupling methodologies (Negishi, Suzuki, Sonogashira etc.).
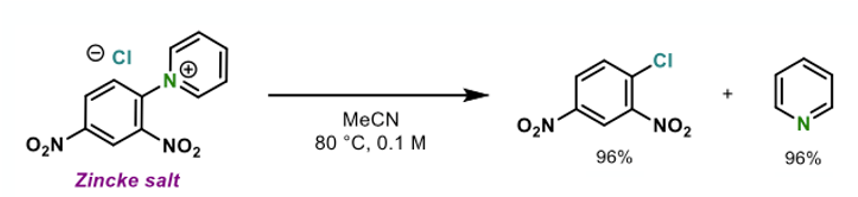
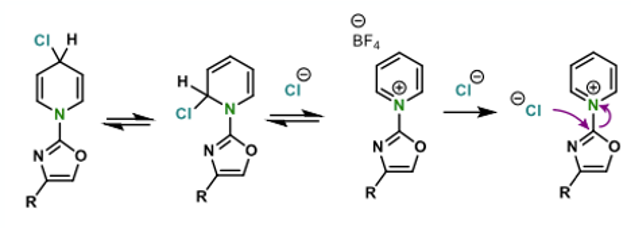
The idea behind this approach came from the key observation that so called “Zincke salts”, formed by reaction of the 1-chloro-2,4-dinitro compound with pyridine, will revert back to staring materials upon heating in solution (Figure 3).
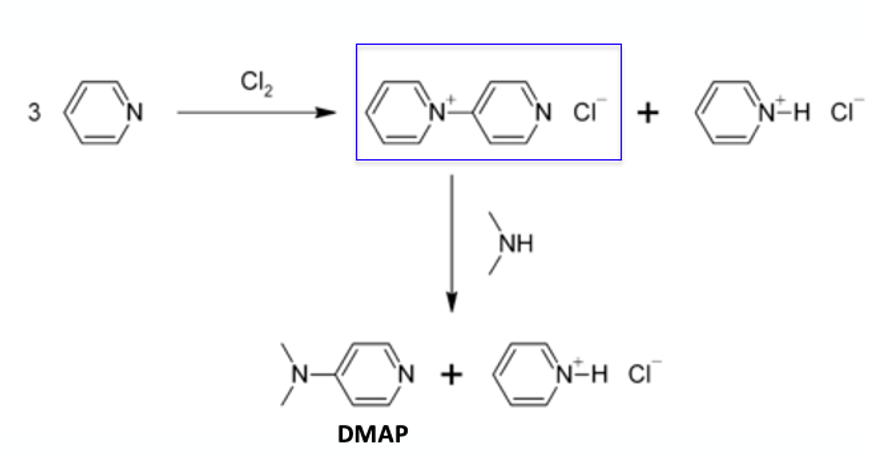
In a similar vein, oxidative dimerization of pyridine is known to generate di-pyridinium salts that again react with a chloride nucleophile generating 4-chloropyridine and pyridine hydrochloride. Actually, a similar process is used to make DMAP through reaction of the di-pyridinium salt with dimethylamine (Figure 4). Also, for anyone thinking of taking our chemical development course- I’ve just given you a big clue to solving one of the workshop problems. Your welcome!
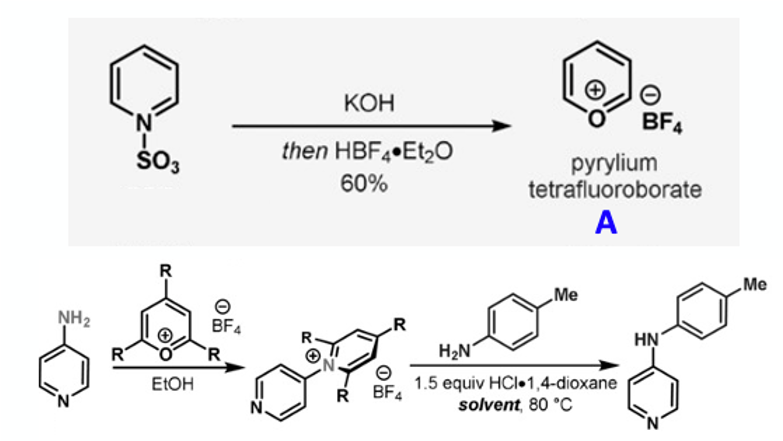
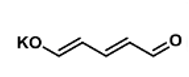
Historical work by the Cornella group demonstrated chemoselective reaction of a pyrylium salt reagent (A) with amines and Zincke-type reactivity of the resulting azines (Figure 5). Pyrylium tetrafluoroborate is now commercially available (CAS: 80279-50-1) and is made from pyridine sulfurtrioxide complex via the scheme shown in Figure 5. Reaction of pyridine sulfurtrioxide with KOH generates glutacon dialdehyde potassium salt (Figure 6). This is treated, in a separate step, with tetrafluoroboric acid to give the corresponding pyrylium salt. According to Cornella this is a bench stable, “rock-like” solid that shows no thermal activity in the DSC below 250°C and is “non-explosive”. I’m not sure if this means it’s been formally tested or if it’s just not blown up the lab (to date).
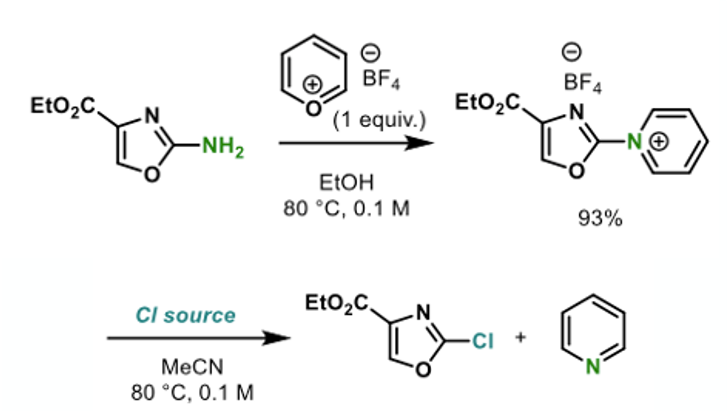
Based on these observations (and their earlier work of pyrylium salt reactivity), a test substrate (ethyl 2-aminooxazole-4-carboxylate) was converted to the pyridinium tetrafluoroborate and various sources of chloride were explored to effect conversion to the aryl chloride (Figure 7). Most chloride sources that were tried (and used in a molar excess) seemed to work (ether/HCl, MgCl2, TMS-Cl, nBuN+Cl–), with elevated temperatures required in most cases. The reaction using MgCl2, carried out as a single pot procedure without isolation of the pyridinium salt, gave 90% yield of the chloro product (2 eqv MgCl2, 0.1M CH3CN, 120°C, 16hrs).
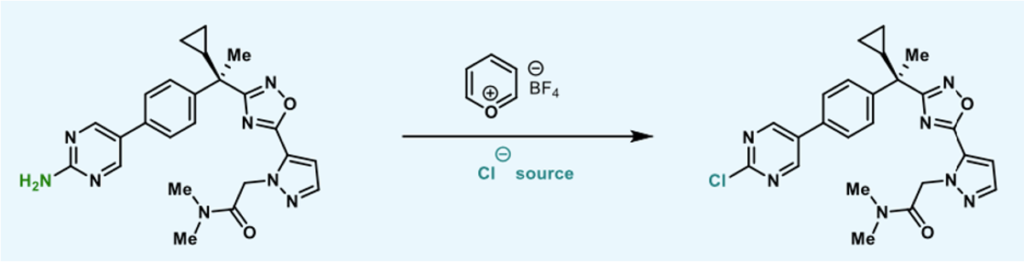
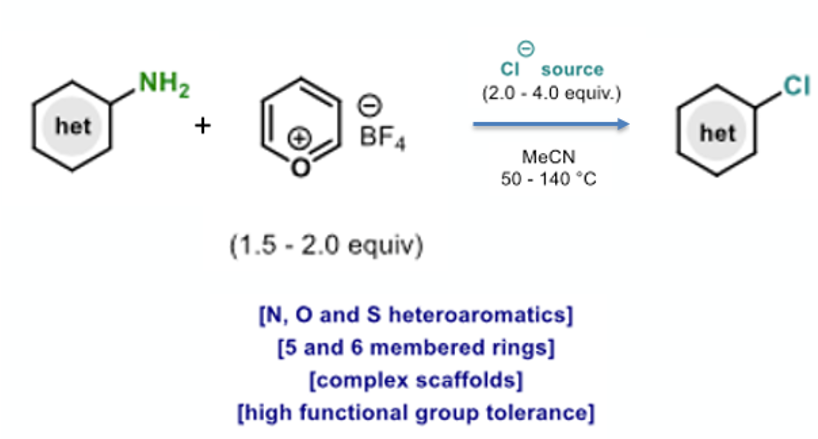
The scope of the reaction was thoroughly explored with a wide range 2- and 4- aminoheterocycles (Figure 9). The team exemplified over 20 distinct types of heterocyclic motifs, including both 5- and 6-membered rings containing N, O and S. The protocol showed broad functional group tolerance enabling late-stage functionalisation of pharmaceuticals,agrochemicals and natural products. Examples include a paroxetine analogue (59%), fipronil (83%) and adefovir (57%). The last two examples gave no chlorinated product when run under standard Sandmeyer conditions (Figure 10).5 Functionalization of the pyrimidine moiety in BI665915 (a BI intermediate, Figure 2) gave a 37% yield of chlorinated product, despite the weak N-O bond in the oxadiazole ring. There are plenty of kinase inhibitors out there in corporate compound collections that have an aminoheterocycle as a conserved ATP hinge-binding fragment in their structure. This methodology gives some potential for conversion of the NH2 to a halide under very mild conditions, producing a useful vector to build out and explore chemical space in binding to known (and unknown) biological targets.6
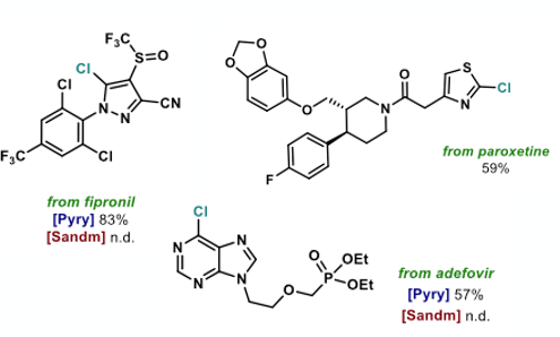
A few bromo- examples are included in the examples described in the paper (using LiBr or MgBr2 under the same reaction conditions), however attempts to introduce fluoride ion resulted in ring-opening of the pyridinium ring.
The authors speculate that chloride (or bromide) attack at the C-2 or C-4 positions of the pyridinium ring is reversible and it is the irreversible attack at the ipso-position that finally generates product (Figure 11).
So there we have it. A great looking piece of chemistry. The end of the Sandmeyer reaction? Most certainly not. But a milder method for achieving the same functional interconversion? – absolutely. Congrats to the Cornella team- I hope to see it in print very soon.
References:
- Deaminative chlorination of aminoheterocycles: J. Cornella et al, 2021: https://chemrxiv.org/articles/preprint/Deaminative_Chlorination_of_Aminoheterocycles/14262671
- Selective functionalization of aminoheterocycles by a pyrylium salt: Cornella et al, Angew. Chem. Int. Ed. 2018, 57, 11035 –11039.
- Recent applications of arene diazonium salts in organic synthesis: J. Wang &G. Dong et al, Org. Biomol. Chem. 2013, 11, 1582-1593; W. Su et al, Recent progress in the use of Vilsmeier-type reagents. Prep. Proced. Int. 2010, 42, 503-555.
- An organic chemist’s guide to nitrosamines: Their structure, reactivity, and role as contaminants: T. Swager et al, Org. Chem. 2021, 86, 3, 2037–2057.
- Sandmeyer conditions: aminoheterocycle (1 equiv.), NaONO (1.1 equiv.); HClaq 37% (0.2 M), 0 °C, 15 min; then, CuCl (1.3 equiv.), 25 °C, 3 h.
- An overview of late-stage functionalization in today’s drug discovery: T. Reekie & M. Kassiou et al, Opin. Drug. Disc. 2019, 14, 1137-1149.








