Oxazoles are relatively common heterocyclic rings, but are not always the easiest rings to prepare by de novo synthesis. Two recent papers describe new methods for the preparation of these compounds1,2.
In the first paper1, a heterogeneous gold catalyst (MCM-41-Ph3PAuNTf2) is used prepare oxazoles from an acetylene, a nitrile with the oxygen atom coming from 8-methylquinoline N-oxide (E):
![]()
There are 24 examples with yields varying from 61-92%, with one other example at 52%.
The second method2 uses an iodine (III) catalyst with the active catalyst being generated in situ via addition of m-chloroperbenzoic acid, the stoichiometric oxidant, and so the method can be considered metal free.
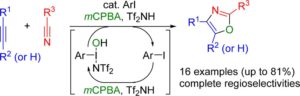
There are 48 examples with yields varying from 24-81%.
- W. Yang, R. Zhang, F. Yi, and M. Cai, J. Org. Chem., 2017, 82, 5204-5211.
- T. Yagyu, Y. Takemoto, A. Yoshimura, V.V. Zhdankin, and A. Saito, Org. Lett., 2107, 19, 2506-2509.








