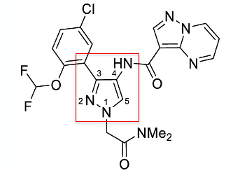
Process chemists at Genentech recently published their work on development of a kilo-scale synthesis of JAK-1 inhibitor- GDC-4379 (Figure 1).1The central heterocyclic core of the molecule consists of a 4-aminopyrazole moiety (elaborated as the pyrazolopyrimidene amide- presumably the inhibitor hinge binding fragment in the kinase active site) with an additional aryl substituent at C-3 and an N,N-dimethylacetamide capping group at N-1.
The original preparation was a 6-step synthesis from commercially available starting materials with an overall yield of 10%. In this early route the key central 4-aminopyrazole fragment was introduced using SEM-protected 4-nitro-1H-pyrazole which underwent a low-yielding (50%) C-H arylation at C3 followed by a low-yielding iron/ammonium chloride reduction of the nitro group (49%, Scheme 1).
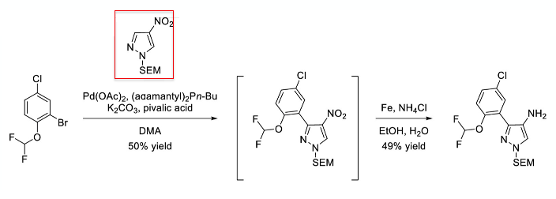
In the paper they comment, amongst other things, on the low yield for this sequence and the use of the SEM- protecting group. SEM-chloride is a particularly nasty reagent. If I were evaluating this route, nitropyrazole stability would be a major concern for me. A quick google search turned up a paper entitled “Thermal stability and detonation characters of nitro substituted derivatives of pyrazole”.2 Something to strike fear into the heart of any process chemist. The nitrogen content of unsubstituted pyrazole is 37%. Increasing the nitrogen/oxygen content by nitration of the pyrazole ring generates high energy-density compounds that would certainly warrant further calorimetric investigation. In “our” case the SEM group, adding additional mass, would most likely increase stability, but having said that preparing and handling large volumes of this intermediate would not, I suspect, be ideal.
Historical point of interest- apparently, according to the nitropyrazole paper, pyrazole itself was first synthesised in 1898 from acetylene and diazomethane. Throw in a nitration and we must have a contender for the most hazardous synthetic sequence to prepare a simple aryl heterocycle.
The nitropyrazole stability paper by Liu is based on calculation of heats of formation, bond orders and bond dissociation energies. It was published in the journal “Molecular Physics” which I confess I never even knew existed. In the paper they also use the term “oxidisation”. Apparently the term oxidation hasn’t yet made it into the physicists’ vocabulary.
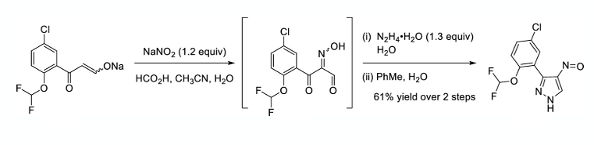
Anyhow- I digress. Having concluded the early synthesis of GDC-4379 was unsuitable for further scale-up they approached the synthesis of the required aminopyrazole using a different bond disconnection, with the C-3 aryl substituent already in place. Claisen condensation of the C-3 requisite acetophenone derivative with ethyl formate gave an isolable sodium enolate (76% yield). Oxamination with sodium nitrite in the presence of formic acid gave the required dielectrophile and a solution of this intermediate was telescoped into a condensation/cyclisation reaction with hydrazine hydrate to furnish the nitrosopyrazole in 61% yield over the two steps. (Scheme 2). The oxime intermediate proved unstable in solution over extended periods of time, hence the need for rapid processing and no isolation step.
The key step in this new synthesis of the aminopyrazole was reduction of the nitroso group to the corresponding amine. Simple hydrogenation over Pd/C was messy- most likely due to competing protodehalogenation. Azoxy impurities were also detected in the crude material. The answer was to use sodium borohydride mediated reduction in the presence of a catalytic amount of suitable copper salt- a novel reduction process, albeit very similar to the process described by Hanaya (below). The catalyst presumably generates a copper hydride species that turns over the borohydride and is responsible for the stochiometric reduction.3 A similar process for nitro-reduction- using Cu(acac)2 in combination with sodium borohydride- was reported by Hanaya in 1979.4 In the GDC-4379 synthesis CuCl proved the best catalyst (7 eqv NaBH4/3 mol% catalyst, EtOH/THF, 72% yield, 42Kg scale, Scheme 3).
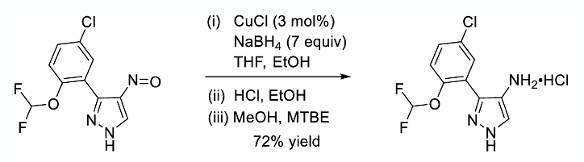
The solvent system, and in particular the combination of solvents at different stages of the reaction were important. NaBH4 reacts with ethanol and the reaction rate is accelerated in the presence of CuCl. In order to reduce the amount of NaBH4 required for the reaction, a solution of the nitroso substrate in EtOH was added to a mixture of NaBH4/CuCl in THF over 2hrs to maintain a dose-controlled process.
The team completed the synthesis without the pyrazole requiring protection- an aspirational achievement worthy of Phil Baran’s paradigm.5
A good example here of re-designing a synthesis for larger scale production and developing some new synthetic approaches on the way.
One thing I’ll overlook here is the 248Kg of Freon 22 (chlorodifluoromethane) used to difluoromethylate the phenol, as described in the experimental section of the paper. CFC’s hammer the ozone layer and that gas is a particularly bad actor. I have no moral high ground here- I used to work on a site that manufactured the stuff.
See you next time.
References:
- Efficient, protecting group free kilogram-scale Synthesis of the JAK1 Inhibitor GDC-4379, A. Stumpf et al, Org. Process Res. Dev. 2021, 25, 2537-2550.
- Thermal stability and detonation characters of nitro substituted derivatives of pyrazole, L. Liu et al, Molecular Physics,2020, 118, DOI: 1080/00268976.2019.1708491
- Coinage metal hydrides: synthesis, characterization, and reactivity, J. Sadighi et al, Chem. Rev. 2016, 116, 8318-8372.
- Reduction of aromatic nitro-compounds to amines with sodium borohydride-copper(II) acetylacetonate, K. Hanaya et al, Chem. Soc., Perkin Trans. 1 1979, 2409−2410.
- Protecting-group-free synthesis as an opportunity for invention, P. Baran et al, Chem. 2009, 1, 193−205.








