Two recent papers in Science have described Iridium catalysed C-H borylation of alkyl C-H bonds.
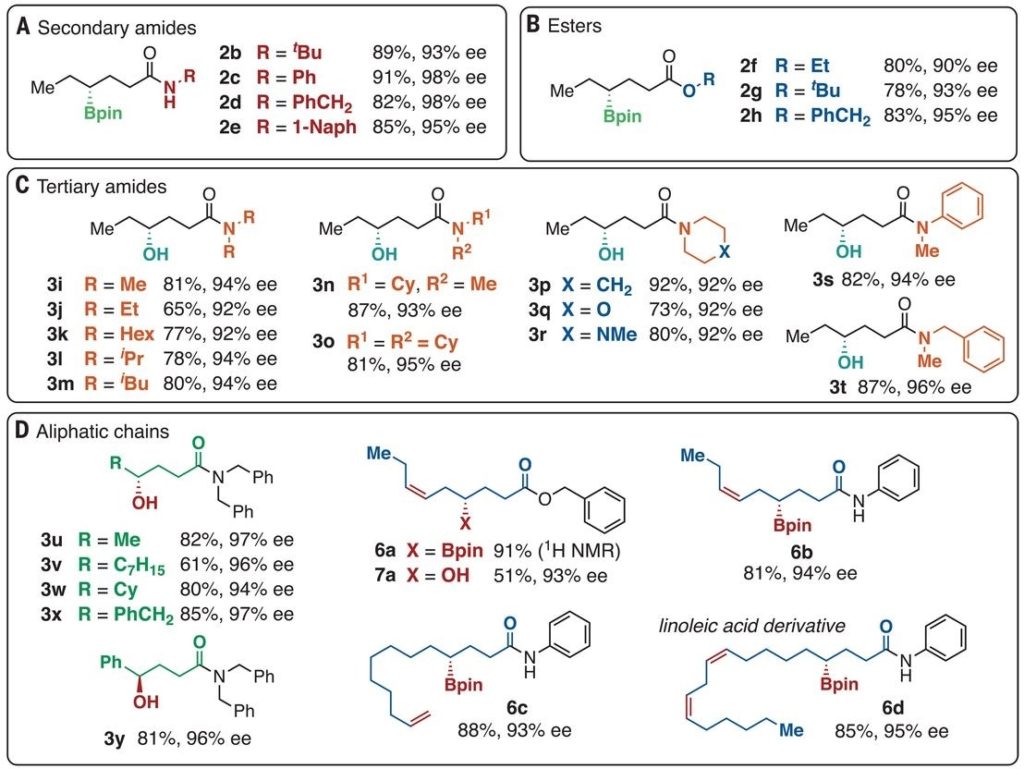
The first paper1 reports a C-H borylation of primary C-H bonds. Initial experiments showed that dibutyl ether (used as substrate and solvent) could be converted to BuO(CH2)4Bpin and similarly THF could be borylated in the 3-position with 2-methylphenanthroline (2-mphen) as ligand giving a 40-fold rate increase when compared with more conventional ligands (2,9-dmphen and 3,4,7,8-tmphen). This allowed the reaction out be carried out with the substrate as the limiting reagent. The borylation of dodecane in cyclooctane at 100°C gave a mixture of products borylated at one or both terminal positions (with a 60:1 ratio of dodecane borylation compared to cyclooctane borylation).
The reaction needs to be carried out with a nitrogen sweep to remove the HBpin by-product which hinders the desired reaction. In fact, HBpin disproportionates with B2pin2 to form B2pin3 and BH3, and it is BH3 that is removed and that drives the disproportionation reaction. The reaction scope is pretty wide (see figure 1 below) although there can be selectivity issues if there are 2 methyl groups in the molecule. If the 2 methyl groups are identical, as in dodecane, over reaction is a problem. Alternatively, if there are 2 different methyl groups in the substrate there can be regioselectivity problems unless there is a big difference in the steric hindrance around the 2 groups.
Figure 1: Scope of the borylation of CH3 groups
The second paper describes an enantioselective borylation at the g-position of alkyl esters and amides. Again an Iridium catalyst is used but in this case there are 2 ligands. On is a so-called receptor ligand which has a urea group which binds the substrate so that the g-H-atom on the substrate is set up for activation but he metal catalyst and the chiral ligand ensures the boron is delivered enantioselectively – see Figure 2.
Figure 2: Remote C-H borylation of carboxylic acid derivatives
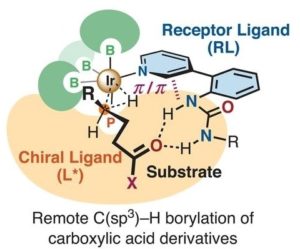
Figure 3 shows the scope of the reaction.
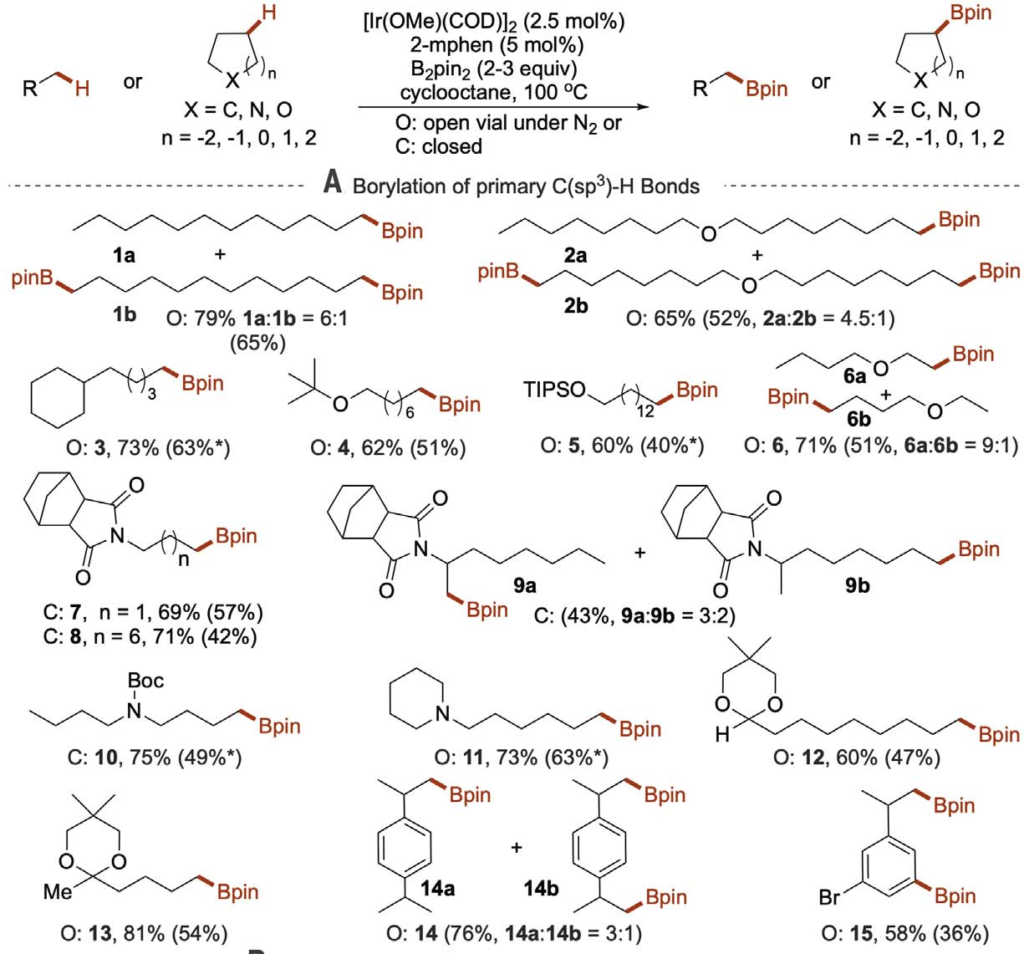
Figure 3 g-Borylation of esters and amides
- Oeschger, B. Su, I. Yu, C. Ehinger, S. He and J. Hartwig, Science, 2020, 368, 736-741.
- L. Reyes, M. Sato, T. Iwai and K. Suzuki, Science, 2020, 369, 970-974.








