Increasing multidrug resistance to antimicrobial agents, particularly in Gram-negative bacteria, is a significant global healthcare challenge. Carbapenem-resistant enterobacteriaceae (CRE) has been declared as one of the most urgent drug-resistant threats in the United States. Much like the cephalosporin B-lactams in the 1980’s, heavy clinical reliance on carbapenem b-lactam antibiotics over the past few decades has driven the emergence of resistant bacteria that are able to engineer serine-based carbapenamase enzymes, in particular Klebsiella pneumoniae carbapenemase(KPC), that specifically targets and degrades the antibiotic’s critical lactam warhead, rendering them inactive. Selective inhibitors of bacterial serine proteases have been used clinically in combination with B-lactam antibiotics to redress the balance.1
One recently approved inhibitor that targets KPC, Vaborbactam (Figure 1), is unusual in that it relies on a central boron atom to immobilise the bacteria’s defensive enzymes, enabling a co-administered antibiotic, Meropenem, to treat complicated urinary tract infections and pyelonephritis.2 The inhibitor was discovered at Rempex Pharmaceuticals, now part of Melinta Therapeutics. Following FDA approval in August 2017 the combination therapy is marketed as Vabomere.3 The boron atom is essential in that it forms a covalent bond with the lactamase enzyme’s catalytic serine residue, effectively mimicking the tetrahedral transition state in the acylation or deacylation pathway, and blocks its activity.4
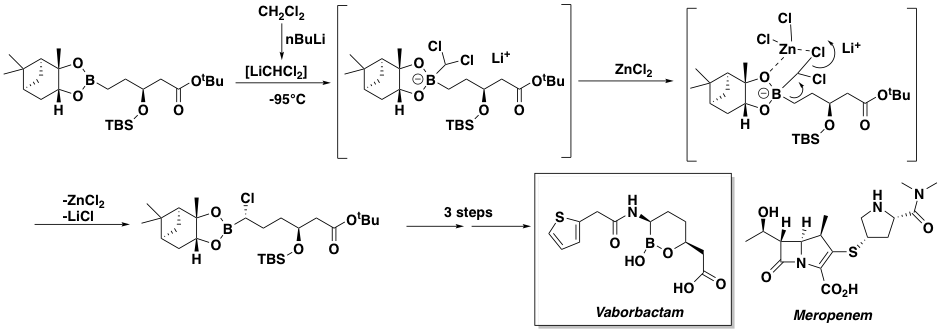
A key step in the synthesis of Vaborbactam is diastereoselective chain homologation of a boronate ester to an a-chloroboronic ester using chemistry originally developed in the 1980’s by Donald Mattison (Figure 1).5 This reaction involves generation of dichloromethyl lithium at low temperatures (typically -95° to -100°C), reaction with a boronate ester to generate a boron “ate” complex and subsequent rearrangement to generate the required chain-extended building block. The homologation is carried out in the presence of zinc chloride which both promotes high levels of diastereoselectivity (due to chelation control in the transition state) and suppresses epimerization of the chlorinated product. The requirement for low temperature generation and processing of the organometallic species stems from its instability at higher temperatures, resulting in decomposition viaformation of a carbene througha-elimination of LiCl.6 This Lithiation-borylation methodology has been further developed by Aggarwal and applied to a number of other complex synthetic targets.7
Scale-up of low temperature organometallic chemistry in batch is intrinsically challenging for a number of reasons. The ability to remove heat from the reactor during exothermic additions at cryogenic temperatures is difficult and often very high dilutions and slow addition rates are required to maintain control. This in turn can decrease efficiency and productivity. Poor mixing, particularly at the point of addition, can also generate localised hot-spots and variable local stoichiometries resulting in formation of impurities and impacting yield and product quality.Continuous processing is frequently used to mitigate problems of this type encountered during batch processing. Flow chemistry is often the preferred approach in scaling up chemistry that utilises highly reactive organometallic reagents such as organolithium or organomagnesium derivatives, including unstable species such as the lithium carbenoids utilized in the Matteson reaction.8 In situ formation and extremely rapid trapping of the organometallic species, before decomposition can occur, is possible under flow conditions but is poorly achieved in a large batch reactor.
The Matteson homologation, used to assemble the pivotal Vaborbactama-chloroboronic ester intermediate (Figure 1), is eminently amenable to a flow chemistry strategy. Based on early pioneering work by a team at Novartis in which millisecond generation and trapping of dichloromethyl lithium at higher temperatures under flow conditions was demonstrated,9 Christian Schuster’s team at Patheon (Thermo Fisher Scientific), in collaboration with a wider group of industry experts, successfully utilized this technology, converting a lab-based model reaction into a continuous manufacturing process run on several hundred-kilogram scale under full cGMP conditions in yields of 97% .10 The corresponding batch process proved unscalable under laboratory conditions. Ultimately several metric tons of Matteson product were prepared in a process validation flow campaign enabling FDA “fast-track” approval and facilitating rapid development of Vaborbactam.
Key to the success of this work was a detailed investigation into the zinc chloride addition and developing conditions to prevent precipitated solids “clogging” the system- a mainstay of flow chemistry processes. In early development work the process flow stream was quenched into a batch vessel containing the pre-cooled zinc Lewis acid. This quickly became a bottleneck for increasing throughput however. Two engineering solutions to this problem were explored, a continuous loop quench (mimicking the batch process) and a continuous stirred tank (CSTR)- based cascade quench. In the loop quench, boronate adduct was fed into a circulating pre-cooled solution of zinc chloride with reaction temperature controlled by heat exchangers. The product solution was pumped out and consumed zinc salt was continuously replenished in the loop reactor to keep the molar ratio constant. In the CSTR approach zinc chloride solution was constantly fed to the pre-cooled vessel, the product solution exiting the CSTR in a cascade system controlled by an overflow device. The loop reactor system was ultimately selected to move forward and enabled the reaction to be run at a higher temperature and with better diastereometric control.
By Leveraging the advantages a continuous process can impart, including increased process control, energy efficiency and reduced processing times, Schuster’s team succeeded in taking a difficult, unscalable organometallic reaction to full-scale production. Another victory in the ongoing battle against the bacteria!
**This article was previously published on Chemical Knowledge Hub** Link Here.
References:
- Evolution of antimicrobial resistance among Enterobacteriaceae (focus on extended spectrum b-lactamases and carbapenamases, J. Lynch et al, Expert Opin. Pharmacother. 2013, 14, 199-210; Three decades of B-lactamase inhibitors, S. Drawz et al, Clin. Microbiol. Rev. 2010, 23, 160-201.
- Meropenem/Vaborbactam: a review in complicated urinary tract infections, S. Dhillon, Drugs 2018, 78, 1259-1270. The first B-lactamase inhibitor approved for clinical use was Clavulanic acid in the 1970’s.
- Discovery of a cyclic boronic acid b-lactamase inhibitor (RPX7009) with utility v’s class A carbapenemases, S. Hecker et al, J. Med. Chem. 2015, 58, 3682-3692; T. Eisenman, FDA approves new antibacterial drug, August 29th2017.
- Synthesis of biologically active boron-containing compounds, H. Zhou et al, Med. Chem. Commun. 2018, 9, 201-211; The versatility of boron in biological target engagement, A. Yudin et al, Nature Chem. 2017, 9, 731-742. The first boron-containing drug, Bortezomib (Velacade), was approved in 2003.
- Homologation of boronic esters to a-chloro boronic esters, Matteson et al,Organometallics, 1983, 2, 1529-1535; a-halo boronic esters: intermediates for stereodirected synthesis, D. Matteson Chem. Rev. 1989, 89, 1535-1551.
- Stability and reactivity control of carbenoids: recent advances and perspectives, V. Gessner, Commun. 2016, 52, 12011-12023.
- Lithiation-borylation methodology and its application in synthesis, V. Aggarwal et al, Acc. Chem. Res. 2014, 47, 3174-3183.
- Flow technology for the genesis and use of (highly) reactive organometallic reagents, R. Luisi et al, Chem. Eur. J. 2020, 26, 19-32; A perspective on continuous flow chemistry in the pharmaceutical industry, M. Baumann & M. Smith et al, Org. Process Res. Dev. 2020, ASAP 10.1021/acs.oprd.9b00524.
- Dichloromethyl lithium: synthesis and application in continuous flow mode, J. Sedelmeier et al, Lett. 2017, 19, 786-789.
- Development of a continuous flow process for a Matteson reaction: from lab to full-scale production of a pharmaceutical intermediate, C. Schuster et al, Org. Process Res. Dev. 2019, 23, 1069-1077.








