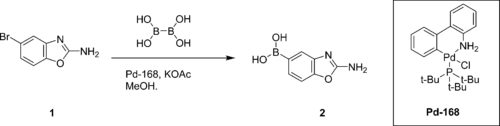
One of the disadvantages of Miyaura borylation is that normally a diboron ester, such as bis(pinacolato)diboron, needs to be used and the resultant coupled ester has to be hydrolysed to its corresponding acid. This leads to poor atom efficiency and longer processing times. Separating the pinacol waste from desired products can sometimes be challenging.
A group from Albany Molecular, Takeda and Carbogen has now found a way to carry out Miyaura borylation using the cheaper tetrahydroxy-diboron (BBA)and applied this to a key step in the manufacture of the Takeda drug TAK-117. BBA is unstable at elevated temperatures so a comprehensive study by 11B NMR and safety evaluation was required before scale up. It was found that addition of ethylene glycol greatly stabilized the process. The reactions are, however, oxygen sensitive and thus control of the dissolved oxygen and the headspace oxygen is needed for efficient reaction and safe scale up. A 47% reduction in cost, compared to using the bis-(pinacolato)diboron, was achieved.
Gurung et al, OPRD, 2017, DOI 10.1021/acs.oprd 6b00345