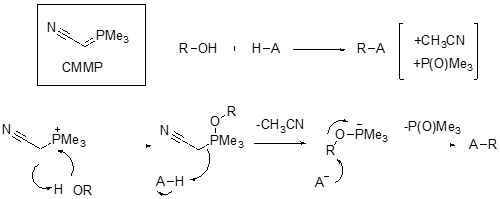
The Mitsunobu reaction is a powerful coupling method for alkylation of various nucleophiles (H-A) with alcohols (R-OH).[1] The classical conditions use a redox system comprising of an azodicarboxylate (such as diethylaziodicarboxylate, DEAD) and a phosphine (normally triphenylphosphine, TPP). The alcohol is essentially activated in situ and coupling occurs with the generation of the phosphine oxide (a significant driving force) and the reduced azo-intermediate. There are a couple of limitations to the reaction- one intrinsic to the chemistry and the other related to process safety. The pKa of the nucleophilic component (H-A) needs to the lower than 11 for the reaction to proceed at a suitable rate. If the Pka is too high (>13) the reaction does not happen at all. The azodicarboxyate reagent is a high energy intermediate and is explosive.[2] The reaction operating temperature usually falls uncomfortably close to the decomposition temperature and process safety concerns lead to alternative synthetic approaches being sought.[3] A paper by Tsunoda, published in 2003 (Chem. Pharm. Bull. 2003, 51(4), 474) describes development of (cyanomethylene)trialkyphosphoranes as potentially safer coupling reagents, designed to mimic the betaine intermediate in the standard DEAD/TPP chemistry with an ylide. A mechanism for the coupling process is shown above. The by-products are acetonitrile and the phosphine oxide.
A key advantage of this methodology is that various nucleophiles with PKa’s in the range 11-23 are facilitated by this chemistry. The reactions can also be carried out at higher reaction temperatures due to increased stability of the CMMP reagent. One drawback, which might be a reason its been less widely adopted, is that CMMP is very sensitive to water and air. The butyl analogue (CMBP) is also described in the paper but is less reactive than the methyl variant. CMMP is commercially available as a solution in THF.[4]
If you have a Mitsunobu reaction in your synthesis it might be worth considering a quick look at this older, underutilized chemistry.
[1] [seminal work: Bull. Chem. Soc. Jpn. 1967, 40, 935]; [Reviews: Org. React. 1992, 42, 335, Chem. Rev. 2009, 109, 2551]
[2] The corresponding diisopropyl analogue (DIAD) is safer to use at scale.
[3] A recent paper by Taniguchi describes evaluation of 2-arylcarboxylates and 2-arylazocarboxamides as thermally stable Mitsunobo reagents, J. Org. Chem. 2018, 83(8), 4712
[4] Sigma Aldrich, 0.5M solution in THF 752940, CAS: 176325-83-0








