Over the last year we have seen a substantive shift in terms of the type / nature of N-Nitrosamines we have seen, moving from the process related small simple dialkyl N-Nitrosamines seen in Valsartan, such as NDMA / NDEA, to API like N-Nitrosamines. Our knowledge is such that we now have a good understanding of the process related risk that can lead to formation of NDMA /NDEA and how to mitigate these. The risk associated with API like N-Nitrosamines is though more complex. The risk associated with API like N-Nitrosamines is one that centres on the scenario where the API concerned is itself a secondary amine. This is estimated to account for something like 20% of all current APIs. In such cases there is a risk of generation of the corresponding N-Nitrosamine. But how? may be the question and related to that where / what is the source of the Nitrosating agent? Water is unlikely, the work of Ashworth et al1 showed that the risk posed by residual nitrite in water, especially that of purified water is negligible. So what is the source? The source is excipients, data published approx. ten years ago 2 showed levels of Nitrite ranging from a few ppm to >100ppm in some excipients. More recent studies managed through a cross industry consortium 3 have put this into better context with the range being more likely sub ppm – 5ppm, however even this is sufficient to, under worst case conditions, present an issue (especially if limits applied to simple dialkyl N-Nitrosamines are unthinkingly applied to large more complex API like N-Nitrosamines).
Can this be resolved? One option is to look to reduce nitrite levels in excipients such as microcrystalline cellulose (MCC) but while this will reduce the risk it is unlikely to eliminate it. What though if it was possible to add a scavenger to ‘mop up’ the nitrosating agent, a sacrificial component that remove the agent of concern. A recent paper, Nanda et al 4 demonstrated that the inhibition of nitrosamine formation in oral solid dosage forms was possible through the addition of a scavenger. Five inhibitors investigated (ascorbic acid, sodium ascorbate, α-tocopherol, caffeic acid, and ferulic acid) showed >80% inhibition when spiked at ∼1 wt.% level.
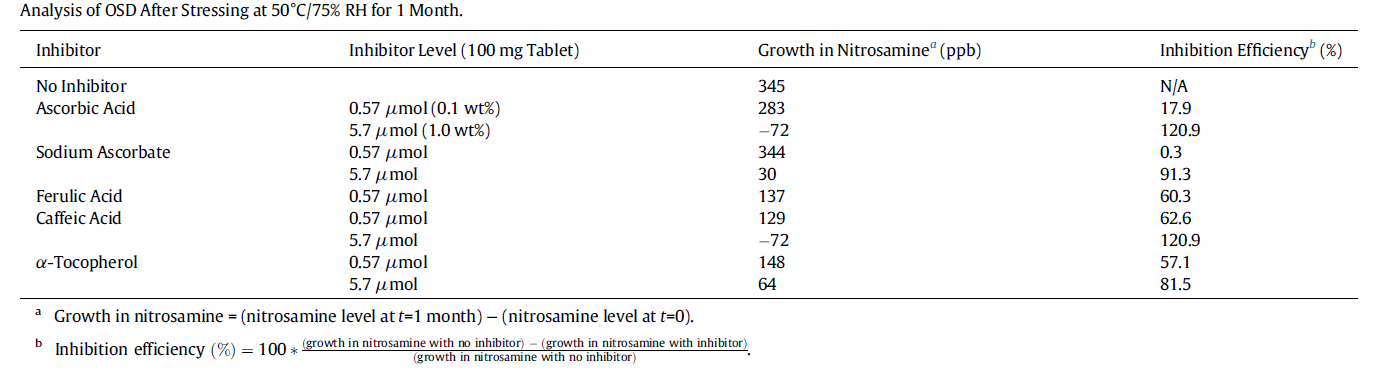
It also showed the potential use of amino acids (glycine, lysine, histidine) as inhibitors of nitrosamine formation in solution.
This work, allied to other advances in our knowledge and understanding of Nitrosamine formation, further enhances our ability to appropriately manage the risk posed by such species in current and future products.
About the Author:
Andrew Teasdale PhD has 30 years’ experience in the pharmaceutical industry as an analytical chemist and within quality assurance and regulatory roles. In his current role he chairs AstraZeneca’s Impurity Advisory Group. Dr Teasdale has published a number of papers relating to mutagenic impurities, N Nitrosamines, extractables and leachables, and other impurity related matters. He is currently the chair of the Extractables and Leachables safety Information exchange (ELSIE) and also led a number of industry expert groups; these include both safety and quality groups within Pharmaceutical Research and Manufacturers of America (PhRMA), European Federation of Pharmaceutical Industries and Associations (EFPIA), Product Quality Research Institute (PQRI) .
Andrew has written an extensive training course programme ‘Practical Management of Impurities and Development of Effective and Comprehensive Control Strategies‘ where he provides a holistic understanding of the challenges and issues associated with implementing an effective control strategy for all impurity types. The next course is available online in March 2022.
References:
- Potential for the Formation of N‑Nitrosamines during the Manufacture of Active Pharmaceutical Ingredients: An Assessment of the Risk Posed by Trace Nitrite in Water. Ian W. Ashworth,* Olivier Dirat, Andrew Teasdale, and Matthew Whiting. Process Res. Dev. 2020, 24, 1629−1646.
- Reactive Impurities in excipients: profiling, identification and mitigation of drug-excipient incompatibility. Wu Y, Levons J, Narang AS, Raghavan K, Rao VM. AAPS PharmSciTech. 2011;12:1248–1263.
- Lhasa Limited. Nitrites in excipient database from Lhasa Ltd. Available at: https://www.lhasalimited.org/news/lhasa-limited-releases-new-data-sharing-initiativenitrites-in excipients/8254.
- Inhibition of N-Nitrosamine Formation in Drug Products: A Model Study. Kausik K. Nanda,*, Steven Tignor, James Clancy, Melanie J. Marota, Leonardo R. Allain, Suzanne M. D’Addio. J Pharm Sci Volume 110, Issue 12, December 2021, Pages 3773-3775








