Aryl boronates are among the most widely used synthetic intermediates in the pharmaceutical and agrochemical industries, both in R&D and commercial manufacture.1 Historically these key building blocks have been made using transition metal (Pd, Cu, Ni) catalysed cross coupling reactions between an appropriate aryl halide (usually bromide, iodide or activated chlorides) and an alkoyboron species.2 Efforts to identify non-transition metal mediated chemistry have thus far been substrate limited to reactive, activated systems using far from generally applicable procedures (UV irradiation in quartz vessels).3 Non-transition metal mediated cleavage of a C-Cl bond in particular is difficult to achieve, with a bond dissociation energy (BDE) of around 97 Kcal/mol.
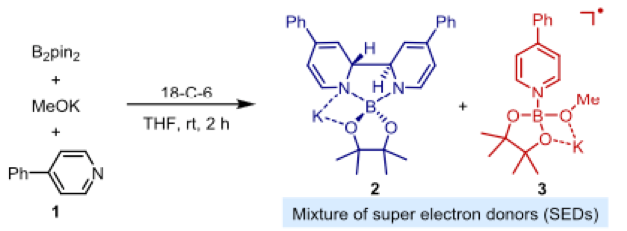
Towards the end of 2018 Zang and Jiao published their work on super electron donors (2, 3) derived from B2pin2, methoxide and catalytic 4-phenylpyridine (1) (Figure 1).4 These complexes promote borylation of aryl iodides and bromides viaactivation of the haloarene and single electron transfer (SET) to form an aryl radical as a key reaction intermediate which undergoes terminal borylation with B2pin2. Activation of chloroarenes however remained a challenge.
Figure 1: Formation of super electron donor (SED) complexes
Attempts to tackle this by improving the reduction potential of the complexes via structural modification of the pyridine catalyst proved unsuccessful. However, the possibility of enhancing the redox potential by photoexcitation of the electron donor/acceptor was viewed as a potential way to tame these difficult substrates.
A recent paper in JACS by the above authors describes extensive investigation of the photochemical properties of complexes (2, 3). In situ generation and photoactivation of complex (2) enabled catalytic borylation of unactivated aryl chlorides (and fluorobenzene!) and is the first example of organocatalytic activation of these substrates under visible (400nm LED) light irradiation (Figure 2) (J. Am. Chem. Soc.2019, 29thMay, article ASAP).
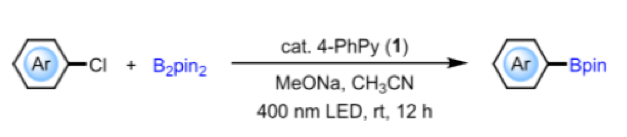
Figure 2: Photochemical borylation of aryl chlorides
The reaction scope and yields appear to be very good (30 examples) with the normal gamut of substituent types, electronic properties and structural motifs. The low reaction temperature provides a big advantage in terms of access to boronates that are thermally labile and difficult to prepare such as 3-pyridyl, 6-indole and 4-anilino (Figure 3a).
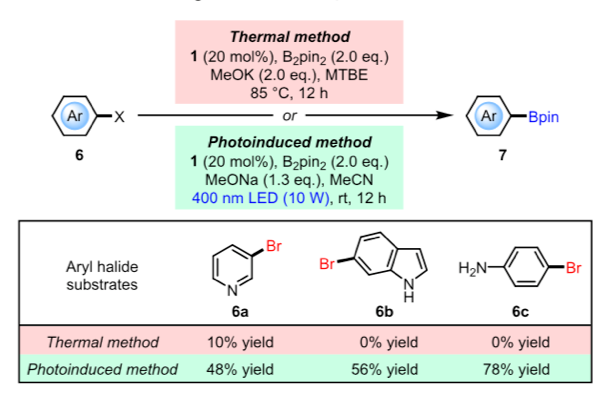
Figure 3: a) Difficult borylation substrates, b) differentially borylated substrates
Another interesting aspect is sequential borylation of a dihaloarene. A reactive halogen such as iodide can be borylayted under thermal conditions leaving a chloro- substituent intact. The latter can then be photoactivated and the molecule differentially borylated (Figure 3b).
Chloroarenes are more ubiquitous than the corresponding bromo or iodo- derivatives and are generally inert, making them easy to store and handle.5 This method provides a novel method for converting them into valuable building blocks under mild, catalytic conditions. This really is the age of organocatalysis and photochemistry.6
References:
- Boronic Acids: Preparation and Applications in Organic Synthesis, Medicine and Materials, 2nd ed.; Hall, D. G., Ed.; Wiley-VCH: Weinheim, Germany, 2011.
- Review: A decade advancement of transition metal-catalyzed borylation of aryl halides and sulfonates: W. Chow et al, RSC Adv. 2013, 3, 12518.
- a) Efficient metal-free photochemical borylation of aryl halides under batch and continuous- flow conditions. K. Chen et al, Chem. Sci. 2016, 7, 3676; (b) Scalable, metal-and additive- free, photoinduced borylation of haloarenes and quaternary aryl-ammonium salts: O. Larionov et al, J. Am. Chem. Soc. 2016,138, 298; (c) Additive-and metal-free, predictably 1, 2- and 1, 3- regioselective, photoinduced dual C−H/C−X borylation of haloarenes: O. Larionov et al, J. Am. Chem. Soc.2016, 138, 8408.
- Super electron donors derived from diboron: L. Jiao et al, Chem. Sci., 2018, 9, 2711.
- Transformations of chloroarenes, catalyzed by transition-metal complexes: V. Grushin et al, Chem. Rev. 1994, 4, 1047-1062.
- Visible light photocatalysis: applications and new disconnections in the synthesis of pharmaceutical agents: J. Douglas et al, Org. Process Res. Dev. 2016, 20, 1134.