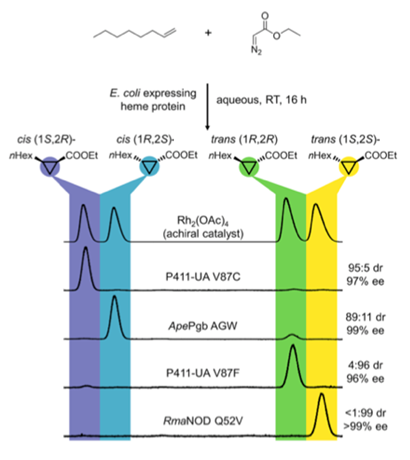
One of the challenges associated with biocatalysis is the resulting stereochemistry is not always easy to predict and more often than not gives the unwanted isomer. The problem is compounded if the substrate has limited steric or electronic components to aid stereocontrol. A way around this problem is to engineer the wildtype protein. A resent paper by the Arnold group (ACS Cent. Sci. 2018, 4, 372) describes sterodivergent cyclopropantion of unactivated terminal olefins via a heme-mediated carbene transfer.[1] A series of promiscuous globin and serine ligated P411 variants of Cyp-P450 were identified and through several rounds of directed evolution mutants were developed that converted unactivated and electron deficient alkene substrates into each of the four possible stereoisomers in high ee (see above). The proteins function in whole E.coli cells under buffered aqueous conditions and is inherently green in concept.[2] No examples of internal olefins were described and turn-over numbers modest. Hydrocarbon feedstocks are abundantly available and this technology offers potential for conversion into valuable chiral building blocks.[3]
[1] Seminal work by the Arnold group on P450 mediated cyclopropanation see Science 2013, 339, 307
[2] For a review of biocatalysis in sustainable chemistry see Chem. Rev. 2018, 118(2), 801
[3] Provivi have done a great deal of work on commercializing enzymatic cyclopropanation and have IP in this area.








