I don’t usually post articles on total synthesis. Their beauty often speaks for itself. However, a recent paper by Zhang and co-workers started me on a trip down memory lane. The paper describes a short, elegant route into the core 1-azabicyclo[4.3.0]nonane structure of the Indolizidine alkaloids (figure 1).1 Together with the structurally related pyrrolizidines (1-azabicyclo[3.3.0]octane), elaborated derivatives of both of these naturally occurring templates are found in up to 3% of the flowering plants on Earth. A very detailed review by Ratmanova describes strategic approaches used to synthesize these compounds.2 The reason Zhang’s paper struck a chord was that my first undergraduate research project (in the early 1990’s) involved the preparation of Indolizidine building blocks used in Gallagher’s synthesis of epi-Castanospermine (Figure 2).3
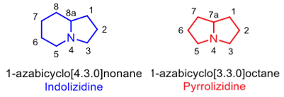
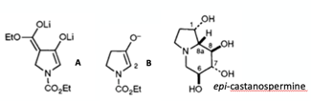
The beta-ketoester dianaion (A) was used at a functional equivalent of the C-2 enolate of N-ethoxycarbonylpyrrolidin-3-one (B) (Figure 2).4 Trying to make the C-2 enolate directly from the ketone was extremely challenging- and believe me I tried. A battle between kinetic and thermodynamic deprotonation was an uphill struggle. Making the ketone substrate was not without its issues too. My first exposure to the wiles of using potassium hydride as a base invoke memories of flaming droplets of hexane and smoking Pasteur pipettes. I didn’t set fire to the lab however. My project also involved taking the amino acid L-alanine through the same Dieckmann cyclisation sequence used to prepare glycine derived (A) to see if it racemised, which of course it did.
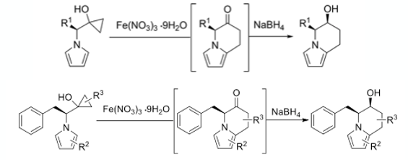
The Zhang paper describes asymmetric synthesis of three indolizidine alkaloids, including the Pharaoh ant pheromone monomorine I (Figure 3). The synthetic approach involves previously unreported ring-opening radical coupling of a cyclopropyl alcohol with an electron rich pyrrole. The key ring-fusion step is driven by generation of a β-keto radical from fragmentation of a cyclopropane ring using a metal salt and radical cyclization onto the 2-position of the pyrrole ring (Figure 3). A review by Njardarson in 2013 describes metal catalysed three- and four- membered ring expansion.5
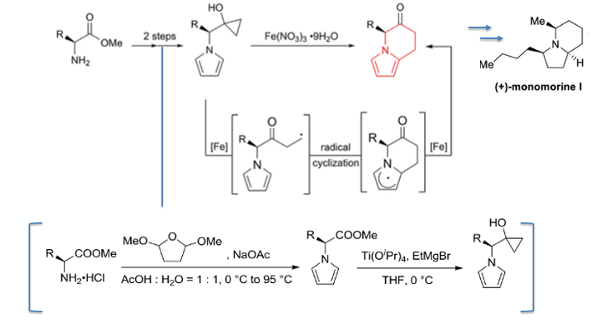
The pyrrole/cyclopropyl substrate(s) were prepared via a Clauson-Kass pyrrole synthesis,6 and Kulinkovich cyclopropanation starting with the appropriate amino acid ester (Figure 3).7
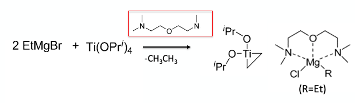
Just as a very brief aside, if competing addition of the (excess) Grignard reagent to the electrophile is a problem in a Kulinkovich-type cyclisation (in place of the required direct addition of titanacyclopropane), an elegant solution developed by Boehringer Ingelheim is the use of stochiometric bis[2-(N,N-dimethylaminoethyl)]ether, which complexes the magnesium, moderates its reactivity and suppresses competing reaction with the electrophile (Figure 4).8
The ring-expanded products were isolated as the corresponding alcohols via diastereoselective reduction of the ketone with NaBH4. The ketones themselves underwent significant erosion of e.e. during silica gel purification. A range of amino acid esters (R1, Figure 5) worked well, as did a range of substituted pyrroles (R2, Figure 5). Direct nitration of the pyrrole nucleus in isoquinoline-fused systems has recently been reported by Cui.11
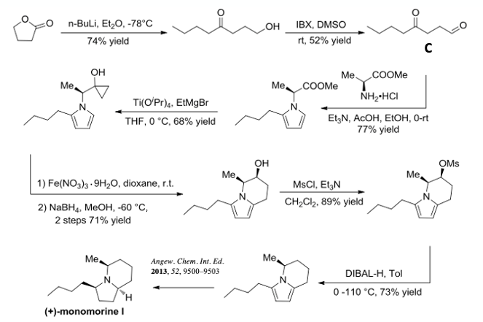
To demonstrate the application of the methodology in a real life example they report a full synthesis of (+)-monomorine I from L-methylalanine and aldehyde (C) (Figure 6). The aldehyde is prepared by ring-opening of the gama-lactone with nBuLi (74% yield) followed by oxidation with IBX/DMSO (52% yield). Pyrrole formation with L-methylalanine followed by Kulinkovich cyclopropanation gave the radical precursor (77% and 68% yield respectively). Treatment with Ferric nitrate gave the fused ring system and ketone reduction gave the alcohol in 71% yield (over 2 steps). Mesylation of the alcohol (89%) followed by DIBAL-H reduction gave the monomorine I precursor in 73% yield. The final pyrrole ring reduction is described in the reference shown (Figure 6). There are several reported syntheses of this natural product, a good summary can be found in the introduction of the report by Stockman and Dawood.12
They didn’t report an influx of Pharaoh ants in the lab- I guess this was music to their ears. A couple of interesting things in here. The paper is well worth a read.
See you next time.
References:
- Arylation of cyclopropanol with pyrrole: asymmetric synthesis of indolizidine 167B, indolizidine 209Dand monomorine I: M. Zhang et al, Lett.2023, 25, 2058–2062
- Strategic approaches to the synthesis of pyrrolizidine and indolizidine alkaloids: N. K. Ratmanova et al, Tetrahedron 2020, 76, 131031; Annulation of pyrrole: application to the synthesis of indolizidine alkaloids: J. A. Smith et al, Tetrahedron 2005, 61, 8226-8230
- N-substituted pyrrolidin-3-ones as heterocyclic building blocks. Enantioselective synthesis of 8-epi- and 1,8,8a-triepi-castanospermine: T. Gallagher et al, J. Chem. Soc., Chem. Commun., 1992, 166-168; Specific enolates from alpha-amino ketones: M. Garst et al, J. Org. Chem. 1980, 45, 2307-2315
- β-Ketoester dianions as regiospecific enolate equivalents for N-substituted pyrrolidin-3-ones: Gallagher et al, J. Chem. Soc., Chem. Commun., 1990, 1047-1048
- Recent advances in the metal-catalyzed ring expansions of three- and four- membered rings: J. Njardarson et al, ACS Catal.2013, 3, 272–286
- Recent advancements in pyrrole synthesis: S. C. Philkhana et al, Synthesis 2021, 53, 1531-1555; A new and high yielding synthesis of unstable pyrroles via a modified Clauson-Kaas reaction: J. A. Smith et al, Tet. Lett. 2006, 799-801; Solvent free synthesis of N-substituted pyrroles catalyzed by calcium nitrate: H. Chaudhari et al, J. Heterocyclic Chem. 2019, 56, 1337
- The Kulinkovich hydroxycyclopropantion reaction in natural product synthesis: M. Brimble et al, Org. Biomol. Chem. 2012, 10, 7649; Stereocontrolled alkylation of unsaturated compounds with alkoxytitanacyclopropane reagents: O. Kulinkovich et al, Chemical Record, 2008, 8, 269
- Process development and pilot-plant synthesis of (s)-tert-butyl 1-oxo-1-(1-(pyridin-2-yl)cyclopropylamino)propan-2-ylcarbamate: studies on the scale-up of kulinkovich–szymoniak cyclopropanation: W. Li et al, Process Res. Dev.2012, 16, 836–839; Addition of Grignard reagents to aryl acid chlorides- an efficient synthesis of aryl ketones: X. J. Wang et al, Org. Lett. 2005, 7, 5593–5595
- Iron-promoted radical reactions: current status and perspectives: A. Gualandi & P Giorgio Cozzi et al, Asian J. Org. Chem. 2017, 6, 1160-1179
- a) Regioselective C-5 nitration of N-protected indolines using ferric nitrate under mild conditions: D. Li et al, Synth. Comm. 2019, 49, 1231-1240; b) Regioselective nitration of anilines with Fe(NO3)3.9H2O as a promoter and nitro source: Y. Gao et al, Org. Biomol. Chem. 2018, 16, 3881-3884
- Nitration of pyrrolo[2,1-a]isoquinolines: H. L. Chi et al, J. Org. Chem. 2023, https://doi.org/10.1021/acs.joc.3c00125
- Stereodivergent total Syntheses of (+)-monomorine I and (+)-indolizidine 195B: R. Dawood & R. Stockman, J. Org. Chem. 2021, 27, 3850-3853; 5-methyl-3-butyl-octahydroindolizine, a novel type of pheromone attractive to Pharaoh’s ants (Monomorium pharaonis(L.): F. Ritter et al, Experientia 1973, 29, 530-531








